
Filed pursuant to Rule 424(b)(1)
Registration No. 333-200033
No securities regulatory authority has expressed an opinion about these securities and it is an offence to claim otherwise.
This prospectus constitutes a public offering of these securities only in those jurisdictions where they may be lawfully offered for sale and therein only by persons permitted to sell such securities. No securities regulatory authority has expressed an opinion about these securities and it is an offence to claim otherwise. Cohbar, Inc. has filed a registration statement on Form S-1 with the United States Securities and Exchange Commission under the United States Securities Act of 1933, as amended, with respect to these securities. See “Plan of Distribution”.
PROSPECTUS
| Initial Public Offering |
December 18, 2014 |

COHBAR, INC.
11,250,000 Units
US $1.00 per Unit
(each Unit consisting of one share of common stock and one-half of one common stock purchase warrant)
This prospectus qualifies the distribution (the “Offering”) of 11,250,000 units (the “Units”) of Cohbar, Inc. (“Cohbar”, the “Company”, “us” or “we”) (the “Offering”), at a price of US$1.00 per Unit (the “Offering Price”). Each Unit is comprised of one share of our common stock, with a par value of US$0.001 per share (each, a “Share” and collectively, the “Shares”) and one-half of one common stock purchase warrant (each whole warrant being a “Warrant”). The Warrants will be created and issued pursuant to the terms of a warrant indenture (the “Warrant Indenture”) dated as of the closing date between us and CST Trust Company, as warrant agent thereunder. Each Warrant will entitle its holder to purchase one share of our common stock at a price per share equal to US$2.00 at any time for up to 24 months after the closing date of the Offering (the “Warrant Expiry Time”), provided that if at any time the volume weighted average trading price of the shares of our common stock is equal to or exceeds US$3.00 per share for twenty (20) consecutive trading days after the date on which our common stock is first traded on the TSX-V, the Company shall have the right and option, exercisable at its sole discretion, to accelerate the expiration time of the Warrants. The Units are being offered in all of the provinces of Canada other than Quebec (the “Eligible Provinces”) pursuant to this prospectus. No offer or sale of Units will be made in the United States or any state, district, commonwealth or territory thereof. Although this prospectus contains a prospectus (the “U.S. Prospectus”) filed with the United States Securities and Exchange Commission (the “SEC”) as part of a registration statement on Form S-1, the Units will not be offered or sold in the United States or any state, district, commonwealth or territory thereof, nor will offers or sales of the Units be made to any person who is a “U.S. person” as defined in Rule 902(k) of Regulation S promulgated under the United States Securities Act of 1933, as amended (the “Securities Act”). The full text of the U.S. Prospectus is included in and forms a part of this prospectus. We have engaged Haywood Securities Inc. (the “Agent”) to act as our agent in connection with the sale of the Units on a commercially reasonable efforts, all or none basis. The
CDN-1
Offering Price of the Units is US$1.00 per Unit, and was determined by negotiation between us and the Agent. See “Plan of Distribution”.
| Minimum offering of 11,250,000 Units |
Price to the Public(1)(2) |
Agent’s Commissions(3)(4) |
Net Proceeds to Cohbar(4) |
|||||||||
| Per Unit |
US$ | 1.00 | US$ | 0.07 | US$ | 0.93 | ||||||
| Offering |
US$ | 11,250,000 | US$ | 787,500 | US$ | 10,462,500 | ||||||
| (1) | Price determined based on negotiation with the Agent. |
| (2) | For the Company’s purposes, US$0.89 of the Offering Price for each Unit will be allocated to each Share and US$0.11 of the Offering Price for each Unit will be allocated to each half Warrant. |
| (3) | We have retained the Agent to solicit subscriptions for the Units on a commercially reasonable efforts basis. As consideration for its services, the Agent will receive: (i) a cash commission equal to 4% of the gross proceeds from the sale of units in the offering to certain specified purchasers and 7% of the gross proceeds from the sale of Units in the offering to all other purchasers; (ii) options (the “Compensation Options”) entitling the agent to purchase a number of units equal to 4% of the number of Units sold under the offering to certain specified purchasers and 7% of the number of Units sold under the offering to all other purchasers for a period of 18 months from the closing date at a price of US$1.00 per Unit; and (iii) an aggregate cash work fee of up to $30,000 payable in three equal monthly installments. The Agent will also be reimbursed for its reasonable fees and expenses including the reasonable legal fees and disbursements of legal counsel to the Agent. This prospectus also qualifies the distribution of the Compensation Options to the Agent. |
| (4) | Before deducting the expenses of the Offering estimated at US$250,000 which, together with the Agent’s commission and fees, will be paid by us out of the proceeds of the Offering. |
There is currently no market through which the Units or the Shares and Warrants comprising the Units may be sold and purchasers may not be able to resell the Shares or Warrants purchased under this prospectus. This may affect the pricing of the securities in the secondary market, the transparency and availability of trading prices, the liquidity of the securities and the extent of issuer regulation. An investment in the Units is subject to a number of risks that should be considered by a prospective purchaser. Investors should carefully consider the risk factors described under “Risk Factors” in the U.S. Prospectus before purchasing the Units. The TSX Venture Exchange (the “TSX-V”) has conditionally approved listing of the shares under the symbol “COB”. Listing of our shares (including the Shares) will be subject to us fulfilling all of the listing requirements of the TSX-V. We do not intend to list the Warrants on any securities exchange.
The Offering Price is in United States dollars. See “Currency and Exchange Rate Information”.
As of the date hereof, we are an “IPO Venture Issuer” (defined under National Instrument 41-101 as an issuer that does not have any of its securities listed or quoted, has not applied to list or quote any of its securities, and does not intend to apply to list or quote any of its securities, on the Toronto Stock Exchange, a U.S. marketplace, or a marketplace outside of Canada and the United States of America other than the Alternative Investment Market of the London Stock Exchange or the PLUS markets operated by PLUS Markets Group plc.). See “Risk Factors” in the U.S. Prospectus.
CDN-2
The following table summarizes the Compensation Options to be granted by us to the Agent pursuant to the Offering:
| Agents’ Position |
Maximum Number of |
Exercise Period |
Exercise Price | |||
| Compensation Options(1) |
787,500 shares of common stock(1) Warrants to purchase up to 393,750 shares of common stock |
18 months from the closing of the Offering | US$1.00 per Unit(2) | |||
| Total Securities under Compensation Options |
1,181,250 shares of common stock |
| (1) | Each Compensation Option entitles the agent to purchase a Unit comprised of one share of common stock and one half of one common stock purchase warrant. |
| (2) | The Compensation Options have an exercise price of US$1.00 per Unit. The warrants included in the Units have an exercise price of US$2.00 per whole share. |
The Agent, as agent on behalf of the Company, conditionally offers the Units qualified under this prospectus, subject to prior sale, if, as and when issued by us and accepted by the Agent in accordance with the conditions contained in the agency agreement referred to under “Plan of Distribution” and subject to the approval of certain legal matters on our behalf by McCullough O’Connor Irwin LLP (as to certain matters of Canadian law), Thorsteinssons LLP (as to certain Canadian federal tax matters), and Garvey Schubert Barer (as to certain matters of U.S. law), and on behalf of the Agent by Wildeboer Dellelce LLP (as to certain matters of Canadian law) and Dorsey & Whitney LLP (as to certain matters of U.S. law). See “Plan of Distribution”.
The financial statements included in this prospectus have not been prepared in accordance with Canadian generally accepted accounting principles or international financial reporting standards (IFRS) and may not be comparable to financial statements of a Canadian issuer. See “Notice to Investors Regarding GAAP”.
The Agent must sell the number of Units that will result in us achieving the minimum gross proceeds in the Offering of US$11,250,000, if any are sold. The Offering will close as soon as practicable after gross proceeds in respect of 11,250,000 Units have been raised. The Agent will hold the funds received in payment for the Units sold in this Offering until the closing of the Offering. No funds shall be released to us until such time as the minimum gross proceeds of US$11,250,000 are raised. If the Offering is not completed on or before March 18, 2015, or such other dates or dates as may be agreed upon by the Company and the Agent, but in any event no later than 90 days after the issuance by the Canadian securities regulators of a receipt for the final prospectus (such actual closing date herein referred to as the “Closing Date”), no Units will be sold and all subscription funds will be returned to subscribers without interest or deduction.
Subscriptions will be received subject to rejection or allotment in whole or in part, and the Agent reserves the right to close the subscription books at any time without notice. Provided that the Offering is subscribed for at least the minimum of 11,250,000 Units, it is expected that the closing of the Offering will occur on or about January 6, 2015, subject to postponement, as the Company and the Agent may agree, to a date not later than the Closing Date.
Potential investors are advised to consult their own legal counsel and other professional advisors in order to assess income tax, legal and other aspects of this investment.
No person has been authorized to give any information other than that contained in this prospectus, or to make any representations in connection with the Offering made hereby, and, if given or made, such information or representation must not be relied upon as having been authorized by us. This prospectus does not constitute an
CDN-3
offer to sell or a solicitation of an offer to buy securities in any jurisdiction or to any person to whom it is unlawful to make such an offer to solicitation in such jurisdiction. Information from other sources, including the Company’s website, is not incorporated by reference in this Prospectus and should not be relied upon by investors.
Cohbar is incorporated under the laws of Delaware in the United States, a foreign jurisdiction. Each of Jon Stern, Jeffrey F. Biunno, Albion J. Fitzgerald and Pinchas Cohen, who have signed the Certificate of the Company attached as CDN-C-1 to this prospectus, and each of our other directors, Marc E. Goldberg and Nir Barzilai, resides outside of Canada. In addition, Marcum LLP, Garvey Schubert Barer, and Dorsey & Whitney LLP, each of whom are experts named in the U.S. Prospectus, are organized under the laws of a foreign jurisdiction outside of Canada. The Company and each of the foregoing officers and directors of the Company has named McCullough O’Connor Irwin LLP of Suite 2600 Oceanic Plaza, 1066 West Hastings Street, Vancouver, British Columbia, V6E 3X1 as its agent for service of process.
Purchasers are advised that it may not be possible for investors to enforce judgments obtained in Canada against any person or company that is incorporated, continued or otherwise organized under the laws of a foreign jurisdiction or resides outside of Canada, even if the party has appointed an agent for service of process.
Unless the context requires otherwise, references to the “Company”, “Cohbar”, “we”, “us”, or “our” refer to Cohbar, Inc.
CDN-4
| CDN-5 | ||||
| CDN-6 | ||||
| CDN-6 | ||||
| CDN-6 | ||||
| CDN-7 | ||||
| CDN-7 | ||||
| CDN-7 | ||||
| CDN-8 | ||||
| CDN-8 | ||||
| CDN-8 | ||||
| CDN-8 | ||||
| CDN-9 | ||||
| CDN-13 | ||||
| CDN-13 | ||||
| A-1 |
Some of the statements contained in this prospectus and the U.S. Prospectus including, without limitation, financial and business prospects and financial outlooks, may be forward-looking statements which reflect management’s expectations regarding future plans and intentions, growth, results of operations, performance and business prospects and opportunities. Words such as “may”, “will” “should”, “could”, “anticipate”, “believe”, “expect”, “intend”, “plan”, “potential”, “continue” and similar expressions have been used to identify these forward looking statements. Examples of such forward-looking statements within the U.S. Prospectus include:
| • | statements regarding anticipated outcomes of research, pre-clinical and clinical trials for our lead peptides and other MDPs; |
| • | expectations regarding the future market for any drug we may develop; |
| • | expectations regarding the growth of MDPs as a significant future class of drug products; |
| • | statements regarding the anticipated therapeutic properties of drug development candidates derived from MDPs; |
| • | expectations regarding our ability to effectively protect our intellectual property; |
| • | statements concerning perceived competitive advantages and our ability to defend competitive advantages; |
| • | expectations regarding our ability to attract and retain qualified employees and key personnel; and |
| • | statements regarding the expected use of the proceeds of the offering. |
These statements reflect management’s current beliefs and are based on information currently available to management. Forward-looking statements involve significant risks and uncertainties, including without limitation, those listed in the “Risk Factors” section of the U.S. Prospectus. A number of factors could cause actual results to differ materially from the results discussed in the forward-looking statements including, but not limited to, changes in general economic and market conditions and the risk factors disclosed under “Risk Factors” in the U.S. Prospectus. Although the forward-looking statements contained in this prospectus and the U.S. Prospectus are based upon what management believes to be reasonable assumptions, management cannot assure that actual results will be consistent with these forward-looking statements. Investors should not place undue reliance on forward-looking statements. These forward-looking statements are made as of the date hereof
CDN-5
and we assume no obligation to update or revise them to reflect new events or circumstances, except as required by applicable laws.
CURRENCY RATE AND EXCHANGE RATE INFORMATION
Our financial results are measured and reported in United States dollars. The following table sets forth, for the periods indicated, the high, low, average and period-end noon buying rates of exchange for one United States dollar in Canadian dollars published by the Bank of Canada. Although obtained from sources believed to be reliable, the data is provided for informational purposes only, and the Bank of Canada does not guarantee the accuracy or completeness of the data. No representation is made that the United States dollar amounts have been, could have been or could be converted into Canadian dollars at the noon buying rate on such dates or any other dates.
| Year Ended December 31 | Period Ended September 30 | |||||||||||||||||||
| 2013 | 2012 | 2011 | 2014 | 2013 | ||||||||||||||||
| Highest rate during period |
Cdn$ | 1.0697 | Cdn$ | 1.0418 | Cdn$ | 1.0604 | Cdn$ | 1.1107 | Cdn$ | 1.0409 | ||||||||||
| Lowest rate during period |
$ | 0.9839 | $ | 0.9710 | $ | 0.9449 | $ | 1.0739 | $ | 0.9921 | ||||||||||
| Average rate during period |
$ | 1.0299 | $ | 0.9996 | $ | 0.9891 | $ | 1.0944 | $ | 1.0236 | ||||||||||
| Rate at the end of period |
$ | 1.0636 | $ | 0.9949 | $ | 1.0170 | $ | 1.1200 | $ | 1.0303 | ||||||||||
On December 17, 2014, the noon buying rate of the Bank of Canada was US$1.00 = Cdn$1.1629. Unless otherwise specified, all references to “dollars”, “US$” or “$” in this prospectus are to United States dollars and references to “Cdn$” in this prospectus are to Canadian dollars.
NOTICE TO INVESTORS REGARDING GAAP
The financial statements included in the U.S. Prospectus have been prepared in accordance with accounting principles generally accepted in the United States, which differ in certain material respects from Canadian generally accepted accounting principles and international financial reporting standards (IFRS). As we will become an “SEC issuer” (as such term is defined in National Instrument 52-107 of the Canadian Securities Administrators) as a result of our U.S. Registration Statement on Form S-1 becoming effective with the SEC, we are not required to provide, and have not provided, a reconciliation of our financial statements to Canadian generally accepted accounting principles.
Upon the receipt for this prospectus being issued by the securities regulatory authorities in the Eligible Provinces we will become a reporting issuer under the securities laws of such jurisdictions. Pursuant to the rules of the securities regulatory authorities of such provinces, we (or, in the case of insider reporting, our insiders) will be required to satisfy the requirements of the laws of such jurisdictions relating to continuous disclosure, proxy solicitation and insider reporting. These laws generally permit us to comply with certain informational requirements applicable in the United States instead of the continuous disclosure requirements normally applicable in such Canadian jurisdictions, provided that the relevant documents are filed with the securities regulatory authorities in the relevant Canadian jurisdictions and are provided to security holders in Canada to the extent and in the manner and within the time required by applicable U.S. requirements.
ROADSHOW MARKETING MATERIALS
The Roadshow Marketing Materials attached as Annex 1 to the Amended and Restated Preliminary Prospectus were filed on SEDAR (www.sedar.com) on November 28, 2014. Subsequent to that filing the Company
CDN-6
increased the size of the Offering from 10,000,000 Units, for gross proceeds of US$10,000,000, to 11,250,000 Units, for gross proceeds of US$11,250,000. As a result, certain statements in the Roadshow Marketing Materials concerning the size of the Offering, the holdings of certain principal shareholders, the gross proceeds to be received by the Company pursuant to the Offering, the funds available to the Company subsequent to the Offering and the use of those funds have been amended to reflect the increase in the size of the Offering.
Those amendments are included in the Roadshow Marketing Materials attached as Annex 1 to this final prospectus. In addition, a revised template version of the Roadshow Marketing Materials can be viewed under the Company’s profile on www.sedar.com.
Cohbar is incorporated under the laws of Delaware in the United States, a foreign jurisdiction. Each of Jon Stern, Jeffrey F. Biunno, Albion J. Fitzgerald and Pinchas Cohen, who have signed the Certificate of the Company attached as CDN-C-1 to this prospectus, and each of our other directors, Marc E. Goldberg and Nir Barzilai, resides outside of Canada. In addition, Marcum LLP, Garvey Schubert Barer, and Dorsey & Whitney LLP, each of whom are experts named in the U.S. Prospectus, are organized under the laws of a foreign jurisdiction outside of Canada. The Company and each of the foregoing officers and directors of the Company has named McCullough O’Connor Irwin LLP of Suite 2600 Oceanic Plaza, 1066 West Hastings Street, Vancouver, British Columbia, V6E 3X1 as its agent for service of process.
Purchasers are advised that it may not be possible for investors to enforce judgments obtained in Canada against any person or company that is incorporated, continued or otherwise organized under the laws of a foreign jurisdiction or resides outside of Canada, even if the party has appointed an agent for service of process.
Like our directors, our officers and certain of the experts named in the U.S. Prospectus reside outside of Canada. Furthermore, substantially all of the assets of those persons may also be located outside of Canada. It may not be possible for Canadian stockholders to effect service of process within Canada upon such individuals. In addition, it may not be possible to enforce against the Company’s directors and officers or certain of the experts named in the U.S. Prospectus judgments obtained in Canadian courts predicated upon the civil liability provisions of applicable securities legislation in Canada.
AUDITORS, TRANSFER AGENTS & REGISTRARS
Our auditors are Marcum LLP, an independent registered public accounting firm, located in New York, New York.
The main transfer agent and registrar for our common stock is CST Trust Company in Vancouver, British Columbia, and the co-transfer agent and co-registrar for our common stock is American Stock Transfer & Trust Company, LLC in New York, New York. The agent and registrar for our warrants is CST Trust Company in Vancouver, British Columbia.
For a description of our business and the three year history of our business please see the description of our business contained under the heading “Business” beginning at page 49 of the U.S. Prospectus. In addition to historical information, the information disclosed under the heading “Business” in the U.S. Prospectus may include forward looking statements that involve risks, uncertainties and assumptions. Our actual results and the timing of events could differ materially from those anticipated in these forward looking statements as a result of a
CDN-7
variety of factors including those discussed in “Risk Factors” beginning at page 11 of the U.S. Prospectus and elsewhere in the U.S. Prospectus. See discussion under “Forward-Looking Statements” elsewhere in this prospectus and page 27 of the U.S. Prospectus.
For a description on use of proceeds of the Offering see “Use of Proceeds” beginning at page 30 of the U.S. Prospectus.
We have entered into an agency agreement with the Agent dated as of December 18, 2014 with respect to the Units being offered by us (the “Agency Agreement”). For a description of the terms of the Agency Agreement and the distribution of the securities offered under this prospectus see “Plan of Distribution” beginning at page 100 in the U.S. Prospectus.
In the past 12 months, shares of our common stock or securities convertible or exercisable for shares of our common stock have been issued by us as follows:
| Date of Issuance |
Nature of Securities Issued |
Number of Shares of Common Stock Issued or Issuable |
Issue Price or Exercise Price Per Share of Common Stock |
Aggregate Issue Price or Exercise Price |
||||||||||
| January 2014 |
Convertible Promissory Notes | 420,000 | US$ | 0.50 | US$ | 210,000 | ||||||||
| January 2014 |
Warrants | 20,946 | US$ | 0.50 | US$ | 10,473 | ||||||||
| April 2014 |
Series B Preferred Stock | 5,100,000 | US$ | 0.50 | US$ | 2,550,000 | ||||||||
| April 2014 |
Warrants | 797,075 | US$ | 0.26 | US$ | 207,240 | ||||||||
| April 2014 |
Options | 1,061,248 | US$ | 0.26 | US$ | 275,924 | ||||||||
| June 2014 |
Series B Preferred Stock | 100,000 | US$ | 0.50 | US$ | 50,000 | ||||||||
| July 2014 |
Warrants | 100,000 | US$ | 0.26 | US$ | 26,000 | ||||||||
| August 2014 |
Series B Preferred Stock | 200,000 | US$ | 0.50 | US$ | 100,000 | ||||||||
| November 2014 |
Options | 1,475,687 | US$ | 0.73 | US$ | 1,077,252 | ||||||||
The only material contracts not in the ordinary course of business entered into since the beginning of the last financial year ending before the date of this prospectus, or before the beginning of such financial year where such contract is still in effect, or to be entered into, on or before the closing of the Offering, are as follows:
| (a) | Agency Agreement dated as of December 18, 2014 between Cohbar, Inc. and Haywood Securities Inc. |
| (b) | Form of Warrant Indenture between the registrant and CST Trust Company, as warrant agent. |
| (c) | Investor Rights Agreement dated April 11, 2014 among the Registrant and certain of its stockholders. |
| (d) | Amended and Restated 2011 Equity Incentive Plan to be effective upon closing of the Offering. |
| (e) | Exclusive License Agreement, dated August 6, 2013, between the Company and the Regents of the University of California. |
| (f) | Exclusive License Agreement dated November 30, 2011, between among the Company, the Regents of the University of California, and Albert Einstein College of Medicine of Yeshiva University. |
CDN-8
Copies of these material contracts are attached as exhibits to the U.S. registration statement and are available on www.sedar.com and on www.sec.gov and also may be examined during normal business hours at the offices of our British Columbia legal counsel, McCullough O’Connor Irwin LLP, located at Suite 2600 Oceanic Plaza, 1066 West Hastings Street, Vancouver, British Columbia V6E 3X1, any time during the period of distribution of the Units under this prospectus.
In the opinion Thorsteinssons LLP, special tax counsel to the Company, and Wildeboer Dellelce LLP, the Canadian counsel to the Agent, based on the current provisions of the Income Tax Act (Canada) (the “Tax Act”) and the regulations thereunder, the Shares, if and when listed on a designated stock exchange (which currently includes the TSX-V), will be qualified investments under the Tax Act for trusts governed by registered retirement savings plans (“RRSPs”) registered retirement income funds (“RRIFs”), deferred profit sharing plans, registered education savings plans, registered disability savings plans and tax-free savings accounts (“TFSAs”) (collectively, “Plans”).
Provided the Shares are listed on a designated stock exchange and neither the Company, nor any person with whom the Company does not deal with at arm’s length for the purposes of the Tax Act, is an annuitant, a beneficiary, an employer or a subscriber under, or a holder of, the Plan, the Warrants will also be qualified investments for a trust governed by a Plan.
The Shares are not currently listed on a designated stock exchange and there can be no assurances that the Shares will be listed on a designated stock exchange. Application has been made to the TSX-V for the listing of the Shares, subject to the Company satisfying the conditions of the TSX-V. The Company will rely upon the TSX-V to proceed in this manner to render the Shares issued on the closing to be a qualified investment for Plans at the time of issuance (the “Company’s Reliance”). If the Company’s Reliance is incorrect, the Shares and the Warrants will not be qualified investments for a Plan as set out in the first and second paragraphs of this section.
Notwithstanding that the Shares and Warrants may be qualified investments for a trust governed by a TFSA, RRSP or RRIF, (“Registered Plan”) the holder of a TFSA or an annuitant of a RRSP or RRIF, as the case may be, will be subject to a penalty tax with respect to the Shares or Warrants held in the Registered Plan if such securities are a “prohibited investment”, as defined in the Tax Act, for the Registered Plan. The Shares and Warrants will generally not be a “prohibited investment” for a Registered Plan provided that the holder or annuitant of such account (i) does not have a “significant interest” (within the meaning of the Tax Act) in the Company and (ii) deals at arm’s length with the Company for the purposes of the Tax Act. In addition, the Shares will not be a prohibited investment if the Shares are “excluded property”, as defined in the Tax Act, for Registered Plans.
Prospective holders that intend to hold the Shares and Warrants in a Registered Plan are urged to consult their own tax advisors to ensure that the securities would not constitute a “prohibited investment” in their particular circumstances.
CERTAIN CANADIAN FEDERAL INCOME TAX CONSIDERATIONS
In the opinion of Thorsteinssons LLP, special tax counsel to the Company, and Wildeboer Dellelce LLP, counsel to the Agent, the following is a summary of the principal Canadian federal income tax considerations under the Tax Act generally applicable to purchasers who acquire ownership of Units under the Offering. This summary is applicable only to a purchaser who, at all relevant times, for the purposes of the Tax Act: (i) deals at arm’s length and is not affiliated with the Company; (ii) who acquires and holds the Shares (including any Shares acquired on the exercise of Warrants) and Warrants as capital property; and (iii) who is or is deemed to be a resident of
CDN-9
Canada (a “Canadian Holder”). Shares and Warrants will generally be considered to be capital property to a Canadian Holder unless the Canadian Holder holds such shares in the course of carrying on a business or has acquired them in a transaction or transactions considered to be an adventure or concern in the nature of trade.
This summary does not apply to a Canadian Holder: (i) that is a “financial institution” for purposes of the mark-to-market rules contained in the Tax Act; (ii) that is a “specified financial institution” as defined in the Tax Act; (iii) an interest in which would be a “tax shelter investment” for the purposes of the Tax Act; (iv) in respect of whom the Company is or would be a “foreign affiliate” for the purposes of the Tax Act; (v) that has made a functional currency election under section 261 of the Tax Act; or (vi) that has entered into, or will enter into, a “derivative form and agreement” or “synthetic disposition arrangement” as defined in the Tax Act with respect to the Shares or Warrants.
This summary is based upon the current provisions of the Tax Act and the Regulations thereunder (the “Regulations”) and counsels’ understanding of the current published administrative practices and policies of the Canada Revenue Agency (“CRA”). This summary also takes into account all specific proposals to amend the Tax Act and the Regulations (the “Proposed Amendments”) that have been publicly announced by the Minister of Finance (Canada) prior to the date hereof. No assurance can be given that the Proposed Amendments will be enacted in the form proposed or at all. This summary does not take into account or anticipate any other changes to the law, whether by legislative, governmental or judicial decision or action, nor does it take into account provincial, territorial or foreign income tax legislation or considerations, which may differ from the Canadian federal income tax considerations discussed below.
This summary assumes that at all relevant times, the Company is not, and is not deemed to be, a resident of Canada for the purposes of the Tax Act.
This summary is of a general nature only, is not exhaustive of all possible Canadian federal income tax considerations and is not intended to be, nor should it be construed to be, legal or tax advice to any particular Canadian Holder. Purchasers should consult their own tax advisors for tax advice having regard to their particular circumstances.
Currency Conversion
For the purposes of the Tax Act, all amounts relating to the acquisition, holding and disposition of Shares and Warrants (including dividends, adjusted cost base and proceeds of disposition) must be expressed in Canadian dollars. Amounts denominated in U.S. dollars must be converted to Canadian currency using the Bank of Canada noon rate on the day on which the amount first arose.
Allocation of Cost
The total purchase price of a Unit to a Canadian Holder must be allocated on a reasonable basis between the Share and each one-half of a Warrant to determine the cost of each to the Canadian Holder for purposes of the Tax Act.
Counsel has been advised that the Company intends to allocate US$0.89 of the issue price of each Unit as consideration for the issue of each Share and US$0.11 of the issue price of each Unit as consideration for the issue of one-half of a Warrant and that the Company believes that allocation is reasonable. The Company’s allocation is not binding on the CRA or the Canadian Holder. The cost of each Share comprising part of a Unit acquired by a Canadian Holder will be averaged with the adjusted cost base to the Canadian Holder of all other Shares held at that time to determine the adjusted cost base of each Share to the Canadian Holder.
Exercise of Warrants
The exercise of Warrants will not constitute a disposition of property for the purposes of the Tax Act and, consequently, no gain or loss will be realized by a Canadian Holder upon the exercise of Warrants. Shares
CDN-10
acquired by a Canadian Holder upon the exercise of Warrants will have an aggregate cost to the Canadian Holder equal to the aggregate of the exercise price paid to acquire such Shares and the adjusted cost base to the Canadian Holder of the Warrants so exercised. The cost of each Share acquired by a Canadian Holder upon exercise of Warrants will be averaged with the adjusted cost base to the Canadian Holder of all other Shares held at that time as capital property to determine the adjusted cost base of each such Share to the Canadian Holder.
Expiry of Warrants
The expiry or termination of an unexercised Warrant will result in a capital loss to a Canadian Holder equal to the Canadian Holder’s adjusted cost base of such Warrant immediately before its expiry on termination. See below under “Capital Gains and Capital Losses” below for a general description of the tax treatment of capital gains and losses under the Tax Act.
Dividends
The full amount of dividends received or deemed to be received by a Canadian Holder on the Shares, including the amount of any foreign tax deducted or withheld therefrom, will be included in computing the Canadian Holder’s income. In the case of a Canadian Holder that is an individual, such dividends will not be subject to the gross-up and dividend tax credit rules in the Tax Act. In the case of a Canadian Holder that is a corporation, such dividends will not be deductible in computing the taxable income of the holder.
To the extent that withholding tax is deducted in respect of dividends paid on the Shares, the amount of such tax generally will be eligible for foreign tax credit or deduction subject to detailed rules and limitations under the Tax Act.
Disposition of Shares and Warrants
A Canadian Holder that disposes or is deemed to dispose of a Share or Warrant (otherwise than by the expiry or exercise thereof) will realize a capital gain (or capital loss) equal to the amount by which the proceeds of disposition of the Share or Warrant are greater than (or less than) the aggregate of the Canadian Holder’s adjusted cost base of such Share or Warrant immediately before disposition and any reasonable costs of disposition. See “Capital Gains and Capital Losses” below for a general description of the tax treatment of capital gains and losses under the Tax Act.
Capital Gains and Capital Losses
One-half of any capital gain (a “taxable capital gain”) realized by a Canadian Holder in a taxation year will be included in the Canadian Holder’s income for the year. One-half of any capital loss (an “allowable capital loss”) realized by the Canadian Holder in a year may be deducted against taxable capital gains realized in the year. Any excess of allowable capital losses over taxable capital gains in a taxation year may be carried back up to three taxation years or forward indefinitely and deducted against net taxable capital gains in those other years, to the extent and in the circumstances specified in the Tax Act.
A Canadian Holder that is throughout the relevant taxation year a “Canadian controlled private corporation”, as defined in the Tax Act may be liable to pay an additional refundable tax of 6 2⁄3% on its “aggregate investment income” for the year, which will include taxable capital gains.
The amount of any capital loss arising on the disposition or deemed disposition of any Common Shares by a Canadian Holder that is a corporation may be reduced by the amount of dividends received or deemed to have been received by it on such shares to the extent and under circumstances prescribed by the Tax Act. Similar rules may apply where the corporation is a member of a partnership or a beneficiary of a trust that owns such shares or
CDN-11
where a trust or partnership of which the corporation is a beneficiary or a member is a member of a partnership or a beneficiary of a trust that owns any such shares.
Alternative Minimum Tax
The Tax Act provides for an alternative minimum tax that is applicable to Canadian Holders who are individuals (including certain trusts and estates). This tax is computed by reference to an adjusted taxable income amount. Eighty percent of capital gains (net of capital losses) and the actual amount of taxable dividends (not including any gross-up) are included in adjusted taxable income. Any additional tax payable by a Canadian Holder under the minimum tax provisions may be carried forward and applied against certain tax otherwise payable in any of the seven immediately following taxation years to the extent specified by the Tax Act.
Offshore Investment Fund Property Rules
The Tax Act contains rules which, in certain circumstances, may require a Canadian Holder to include an amount in income in each taxation year in respect of the acquisition and holding of Shares or Warrants, if:
(a) the value of such Shares or Warrants may reasonably be considered to be derived, directly or indirectly, primarily from portfolio investments in: (i) shares of one or more corporations, (ii) indebtedness or annuities, (iii) interests in one or more corporations, trusts, partnerships, organizations, funds or entities, (iv) commodities, (v) real estate, (vi) Canadian or foreign resource properties, (vii) currency of a country other than Canada, (viii) rights or options to acquire or dispose of any of the foregoing, or (ix) any combination of the foregoing (“Investment Assets”); and
(b) it may reasonably be concluded that one of the main reasons for the Canadian Holder acquiring or holding a Share or Warrant was to derive a benefit from portfolio investments in Investment Assets in such a manner that the taxes, if any, on the income, profits and gains from such Investment Assets for any particular year are significantly less than the tax that would have been applicable under Part I of the Tax Act if the income, profits and gains had been earned directly by the holder.
If applicable, these rules would generally require a Canadian Holder to include in income for each taxation year in which such holder holds the Shares or Warrants, an imputed amount determined by applying a prescribed rate of interest to the “designated cost” (as defined for purposes of the offshore investment fund property rules) to the holder of the Shares or Warrants at the end of each month in the year, less the amount of income for the year (other than a capital gain) of the holder from the Shares or Warrants. Any amount required to be included in computing a Canadian Holder’s income in respect of a Share or Warrant under these rules would be added to the adjusted cost base to the holder of such Share or Warrant.
The application of these rules depends, to a large extent, on the reasons for a Canadian Holder acquiring or holding Shares and Warrants. Canadian Holders are urged to consult their own tax advisors regarding the application and consequences of these rules.
Foreign Property Information Reporting
In general, a Canadian Holder that is a “specified Canadian entity” for a taxation year or fiscal period and whose total cost amount of “specified foreign property” (as such terms are defined in the Tax Act) including Shares and Warrants at any time in the taxation year or fiscal period exceeds $100,000 will be required to file an information return for the taxation year or fiscal period disclosing certain prescribed information. Subject to certain exceptions, a taxpayer resident in Canada will generally be a specified Canadian entity.
The reporting rules in the Tax Act relating to specified foreign property are complex and this summary does not purport to explain all circumstances in which reporting may be required. Canadian Holders
CDN-12
should consult their own tax advisors regarding whether they must comply with these reporting requirements.
Securities legislation in certain provinces of Canada provides purchasers with the right to withdraw from an agreement to purchase securities. This right may be exercised within two business days after receipt or deemed receipt of a prospectus and any amendment thereto. In several of the provinces, the securities legislation further provides a purchaser with remedies for rescission or, in some jurisdictions, damages if the prospectus and any amendment contains a misrepresentation or is not delivered to the purchaser, provided that such remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province for the particulars of these rights or consult with a legal advisor.
Attached is the U.S. Prospectus, which forms part of the U.S. Registration Statement on Form S-1, filed with the SEC in connection with the Offering. The U.S. Prospectus forms a part of this prospectus.
The discussion of the material terms and provisions of the Warrants included in the U.S. Prospectus is qualified in its entirety by reference to the detailed provisions of the Warrant Indenture, a copy of which will be available on www.sedar.com and a copy of which may be obtained by contacting us.
CDN-13
PROSPECTUS

COHBAR, INC.
11,250,000 Units
(each consisting of one share of common stock and one half of one common stock purchase warrant)
$1.00 per Unit
We are offering for sale 11,250,000 units at a price of $1.00 per unit. Each unit consists of one share of our common stock and one half of one common stock purchase warrant. Each whole warrant entitles its holder to purchase one share of our common stock at a price equal to $2.00 per share at any time for up to 24 months after the closing of this offering provided that if at any time the volume weighted average trading price of the shares of our common stock is equal to or exceeds $3.00 per share for twenty (20) consecutive trading days after the date on which our common stock is first traded on the TSX-V, the Company shall have the right and option, exercisable at its sole discretion, to accelerate the expiration time of the warrants. The shares of common stock and the warrants underlying the units will be issued separately.
The offering will not be conducted, and no sales of the units in this offering will be made, in the United States or any state, district, commonwealth or territory thereof, nor will offers or sales of the units in this offering be made to any person who is a “U.S. person” as defined under Rule 902(k) of Regulation S promulgated by the United States Securities and Exchange Commission under the Securities Act of 1933 or any other person in the United States.
Our agent, Haywood Securities Inc. (“agent”) will conduct this offering on a “commercially reasonable efforts, minimum offering” basis, which means that the agent will take all commercially reasonable steps to sell the units on our behalf, and must sell 11,250,000 units if any are to be sold. The offering will close as soon as practicable after being fully subscribed. Subscription funds will be held in trust by the agent until closing of the offering. No funds shall be released to us until such time as the minimum gross proceeds of $11,250,000 are received. If the minimum proceeds of $11,250,000 are not received on or before March 18, 2015, we will terminate the offering and the agent will promptly return all subscription funds to investors without interest or deduction.
| Per unit | Total | |||||||
| Public Offering Price |
$ | 1.00 | $ | 11,250,000 | ||||
| Agent’s Commissions |
$ | 0.07 | $ | 787,500 | ||||
| Proceeds to Cohbar (before expenses) |
$ | 0.93 | $ | 10,462,500 | ||||
See “Plan of Distribution” beginning on page 100.
This is the initial public offering of our securities. There is currently no market through which our securities may be sold, and purchasers may not be able to resell the securities purchased under this prospectus. The TSX Venture Exchange (TSX-V) has conditionally approved the listing of our common stock under the symbol “COB”. Listing of our common stock will be subject to fulfilling all of the requirements of the TSX-V. We do not currently intend to list our common stock on any exchange in the United States. We do not intend to list the warrants on any securities exchange. All amounts are in United States dollars unless otherwise stated.
We are an “emerging growth company” under the U.S. federal securities laws and will be subject to reduced public company reporting requirements. Investing in our securities involves substantial risks. See “Risk Factors” beginning on page 11.
Neither the Securities and Exchange Commission (SEC) nor any other securities commission or regulatory authority has approved or disapproved of these securities or has passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is December 18, 2014.
| Page | ||||
| 1 | ||||
| 10 | ||||
| 11 | ||||
| 27 | ||||
| 29 | ||||
| 30 | ||||
| 31 | ||||
| 32 | ||||
| 34 | ||||
| 36 | ||||
| MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
37 | |||
| 49 | ||||
| 64 | ||||
| 72 | ||||
| 79 | ||||
| SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT |
83 | |||
| 85 | ||||
| 91 | ||||
| MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES FOR NON-U.S. HOLDERS OF COMMON STOCK |
95 | |||
| 100 | ||||
| 103 | ||||
| 103 | ||||
| 103 | ||||
| F-1 | ||||
You should rely only on the information contained in this document or to which we have referred you. The prospectus will only be distributed by us and the agent named herein and no other person has been authorized by us to use this document to offer or sell any of our securities.
Until March 19, 2015 (90 days after the commencement of our initial public offering), all dealers that buy, sell, or trade our securities, whether or not participating in our initial public offering, may be required to deliver a prospectus. This delivery requirement is in addition to the obligation of dealers to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of our units, shares of our common stock and warrants and the distribution of this prospectus outside of the United States.
COHBARTM and other trademarks or service marks of Cohbar, Inc. appearing in this prospectus are the property of Cohbar, Inc. Trade names, trademarks and service marks of other companies appearing in this prospectus are the property of their respective holders.
This summary provides an overview of selected information contained elsewhere in this prospectus and does not contain all of the information you should consider before investing in our securities. You should carefully read the prospectus and the registration statement of which this prospectus is a part in their entirety before investing in our securities.
COHBAR, INC.
Overview
We are a research stage biotechnology company committed to applying our scientific leadership in the biology of Mitochondrial-Derived Peptides, or MDPs, to extend the healthy lifespan and transform the lives of patients with major diseases.
Our scientific leadership is centered on the expertise of our founders, Dr. Pinchas Cohen, Dean of the Davis School of Gerontology at the University of Southern California, and Dr. Nir Barzilai, Professor of Genetics and Director of the Institute for Aging Research at the Albert Einstein College of Medicine, and is supported by our co-founders, Dr. David Sinclair, Professor of Genetics at Harvard Medical School, and Dr. John Amatruda, former Senior Vice President and Franchise Head for Diabetes and Obesity at Merck Research Laboratories.
Our founders and co-founders are widely considered to be scientific experts and thought leaders at the intersection of cellular and mitochondrial genetics and biology, the biology of aging, metabolism, and drug discovery, development and commercialization. The scientific research in the areas of mitochondrial genomics and biology, age-related diseases, longevity, metabolism and MDPs underlying our founder’s discoveries and our intellectual property portfolio was conducted by Dr. Cohen, Dr. Barzilai and their academic collaborators with the support of research grants aggregating over $30 million awarded to their respective academic institutions since 2001 by the National Institutes of Health, private foundations, and other grant funding organizations. The multi-disciplinary expertise of our scientific leaders, and their investigations into and knowledge of age-related diseases, has enabled and focused our Company’s research efforts on the mitochondrial genome and its potential to yield peptides, which are biological molecules composed of bonded amino acids, for therapeutic advancement.
Mitochondria are components within the cell that produce energy and regulate cell death in response to signals received from the cell. They are the only cell components, other than the nucleus, that have their own genome. Until recently, scientists believed the mitochondrial genome was simple, containing only 37 genes, and the mitochondrial genome has been left relatively unexplored as a focus of drug discovery efforts. Research by our founders and their academic collaborators has revealed that the mitochondrial genome has as many as 80 distinct new genes that encode peptides, only several of which have been characterized to date. We refer to these as Mitochondrial-Derived Peptides, or MDPs. MDPs influence cellular activities by acting as messengers between cells, triggering intra-cellular changes that affect cell growth and differentiation and play a role in metabolism.
MDPs represent a diverse and largely unexplored collection of peptides, which we believe have the potential to lead to novel therapeutics for a number of diseases with significant unmet medical needs. We believe that Cohbar is a first mover in exploring the mitochondrial genome to identify MDPs with potential to be developed into transformative medicines, and that the depth of our scientific expertise, together with our intellectual property portfolio, will enable us to sustain this competitive advantage. By augmenting our scientific leadership and MDP discoveries with drug discovery and development expertise and capabilities, we believe we can identify and develop MDP-based therapeutic candidates that harness MDP cell-signaling mechanisms and unlock the therapeutic potential of this collection of peptides.
1
We are the exclusive licensee from the Regents of the University of California and the Albert Einstein College of Medicine to four issued U.S. patents and four U.S. and international patent applications. Our licensed patents and patent applications are directed to compositions comprising MDPs and MDP analogs and methods of their use in the treatment of indicated diseases. See “Business – Patents and Other Intellectual Property”.
Our Strategy
We aim to build a multi-product company based on our expertise in MDP biology that, independently or together with strategic partners, discovers, develops and commercializes first- and best-in-class medicines to treat a wide variety of diseases with large unmet medical need. Key elements of our strategy include:
| • | Exploiting our MDP discoveries to date by advancing research and development within our lead programs; |
| • | Continuing to leverage our expertise in MDP discovery to expand our pipeline of research peptides; |
| • | Expanding our intellectual property portfolio relevant to MDP-based therapeutics; |
| • | Supplementing and supporting our founders’ expertise and efforts with additional scientific leadership, staff and facilities; |
| • | Maintaining our competitive, first-mover advantage in the field of MDP-based therapeutics; |
| • | Leveraging relationships with academic partners and contract research organizations (CROs) to advance our research programs; and |
| • | Developing strategic partnerships with larger pharmaceutical companies and other organizations to support our research programs and future development and commercialization efforts. |
Our Lead Peptides
Our research efforts to date have focused on discovering and evaluating our MDPs for potential development as drug candidates. We seek to identify and advance research on MDPs with superior potential for yielding a drug candidate, and ultimately a drug, for which we have a strong intellectual property position. We also seek to take advantage of efficiencies that may be gained should a single MDP drug candidate prove effective for multiple indications. Based on our evaluation of MDPs currently in our research pipeline we are actively engaged in research of four MDPs for potential advancement into drug candidate programs. We believe that the success of one of these possible MDP candidate programs, and further future development into a clinically effective therapeutic drug, while uncertain, could potentially address significant unmet medical needs. Given the age-related risk factors associated with these disease indications, an effective therapeutic drug could offer substantial improvements in the quality of life, longevity, and medical and care cost burden of our aging population.
MOTS-c
MOTS-c is an MDP discovered in 2012 by our founders and their academic collaborators. To date, our laboratory and rodent studies indicate that MOTS-c plays a significant role in regulation of metabolism and we believe a MOTS-c analog has therapeutic potential for Type 2 Diabetes mellitus, as well as other diseases, such as obesity, fatty liver and certain cancers. We intend to advance research on MOTS-c and its analogs as our lead program.
SHLP-6
We and our academic collaborators have discovered several other MDPs with properties related to humanin, which we refer to as small humanin-like peptides, or SHLPs. Of these MDPs, our investigational research of SHLP-6 and its potential for the treatment of cancer is the most advanced. SHLP-6 cancer treatment models
2
conducted both in cell culture and in mice demonstrated suppression of cancer progression via a dual mechanism involving suppression of tumor angiogenesis (blood vessel development) as well as induction of apoptosis (cancer cell death). We consider SHLP-6 as our primary research peptide for the potential treatment of cancer and plan to advance our research on SHLP-6, or a suitable analog, in parallel with our MOTS-c research program
SHLP-2 and Humanin
Humanin, the first MDP to be discovered, has been shown to have protective effects in various disease models, including Alzheimer’s disease, atherosclerosis, myocardial and cerebral ischemia and Type 2 Diabetes. Humanin levels in humans have been shown to decline with age, and elevated levels of humanin together with lower incidence of age-related diseases has been observed in centenarians as well as their offspring.
We also have evidence that another of our MDPs, SHLP-2, as well as certain of our humanin analogs, may be useful in the treatment of Alzheimer’s disease. In vitro experiments have shown SHLP-2 and these humanin analogs to have protective effects against neuronal toxicity, and have demonstrated that SHLP-2 and the humanin analogs are transported through the blood-brain barrier. We consider SHLP-2, humanin and humanin analogs of potential interest for the development of MDP-based treatments for Alzheimer’s disease.
Our Target Indications
Type 2 Diabetes – Type 2 Diabetes is a chronic disease characterized by a relative deficiency in insulin production and secretion by the pancreas and an inability of the body to respond to insulin normally, i.e. insulin resistance. Hyperglycemia, or raised blood sugar, is a common effect of uncontrolled diabetes and over time leads to serious damage to many of the body’s systems, especially the nerves, kidneys, eyes and blood vessels. The World Health Organization reports that over 346 million people worldwide suffer from diabetes, of which 90% is Type 2 Diabetes.
Cancer – Cancer is a generic term for a large group of diseases that can affect any part of the body. One defining feature of cancer is the rapid creation of abnormal cells that grow beyond their usual boundaries, and which can then invade adjoining parts of the body and spread to other organs. This process is referred to as metastasis. Metastases are a major cause of death from cancer. Cancer is a leading cause of death worldwide. The World Health Organization estimates that in 2012 there were 14.1 million new cancer cases diagnosed, 8.2 million cancer deaths and 32.6 million people living with cancer (within 5 years of diagnosis) worldwide. Cancer drugs such as chemotherapy, hormone therapy and other treatments are used to destroy cancer cells. The goal of cancer drugs is to cure the disease or, when a cure is not possible, to prolong life or improve quality of life for patients with incurable cancer. According to the IMS institute for Healthcare Informatics, global spending on cancer treatment was approximately $91 billion in 2013.
Alzheimer’s disease – In the brain, neurons connect and communicate at synapses, where tiny bursts of chemicals called neurotransmitters carry information from one cell to another. Alzheimer’s disrupts this process and eventually destroys synapses and kills neurons, damaging the brain’s communication network. The Alzheimer’s Association® reports that an estimated 5.2 million Americans suffered from Alzheimer’s disease in 2013 and that by 2025 an estimated 7.1 million Americans will be afflicted by the disease, a 40 percent increase from currently affected patients. There is no cure, and medications on the market today treat only the symptoms of Alzheimer’s disease and do not have the ability to stop its onset or its progression. There is an urgent and unmet need for both a disease-modifying drug for Alzheimer’s disease as well as for better symptomatic treatments.
Atherosclerosis – Atherosclerosis is commonly referred to as a “hardening” or furring of the arteries. It is caused by the formation of multiple atheromatous plaques within the arteries. This process is the major underlying risk for developing myocardial infarction (heart attack) as those plaques will either narrow the vessel
3
or rupture, preventing blood flow in the coronary artery to parts of the heart muscle. Heart disease is the leading cause of death for both men and women. Coronary heart disease is the most common type of heart disease, killing nearly 380,000 people annually. Cholesterol lowering drugs are considered the main preventive approach to treat atherosclerosis, however these drugs are estimated to prevent only one-third of incidences of myocardial infarction, and there is significant unmet need for additional therapeutic options.
Risks
Our business is subject to numerous risks, which are highlighted in the section entitled “Risk Factors” immediately following this prospectus summary, including the following:
| • | We are a research stage company and have not identified a drug development candidate. Our efforts to identify or discover potential drug development candidates may be unsuccessful and there can be no assurance that any drug development candidate we identify can be developed into a drug product. Even if we are able to develop a drug product candidate, we may not be successful in obtaining regulatory approval for commercial sale, or if approved, we may not be able to generate significant revenues or successfully commercialize our products. |
| • | It will take several years before we are able to develop potentially marketable products, if at all, and our research and development plans will require substantial additional capital. We may be forced to curtail our research and development programs or cease operations if we are unable to obtain additional funds. |
| • | If we are unable to maintain our existing relationships with leading scientists and/or establish new relationships with scientific collaborators, our drug development programs may be delayed or terminated and we may be unable to successfully develop our potential product candidates. |
| • | The pharmaceutical market is intensely competitive and any drug product for which we obtain regulatory approval may be unable to compete effectively with existing and newly developed therapies. |
| • | The patent positions of biopharmaceutical products are complex and uncertain and we may be unable to effectively develop, protect or enforce our intellectual property. |
Company Information
Our company was formed as a Delaware limited liability company on October 19, 2007. We converted to a Delaware corporation under the provisions of the Delaware Limited Liability Company Act and the Delaware General Corporation Law on September 16, 2009. Our principal executive offices are located at 2265 East Foothill Blvd., Pasadena, CA 91107. Our telephone number is (415) 388-2222. The address of our registered office in Delaware is 160 Greentree Drive, Suite 101, Dover, DE 19904. We maintain an Internet website at www.cohbar.com. The information contained on, connected to or that can be accessed via our website is not a part of, and is not incorporated into, this prospectus and the inclusion of our website address in this prospectus is an inactive textual reference only. We have no subsidiaries. Unless the context requires otherwise, the words “Cohbar”, “we,” “the company,” “us” and “our” refer to Cohbar, Inc.
About this Prospectus
Our board of directors and stockholders approved a 3.6437695-for-1 forward split of our common stock which was effected on April 2, 2014. All references to common stock, preferred stock, options to purchase common stock, stock options, share data, per share data, warrants and related information have been retroactively adjusted where applicable in this prospectus to reflect the forward stock split of our common stock as if it had occurred at the beginning of the earliest period presented.
Unless otherwise specified, all references to “dollars,” “US$” or “$” in this prospectus are to United States dollars and references to “Cdn$” in this prospectus are to Canadian dollars.
4
The Offering
| Securities offered by Cohbar |
11,250,000 units; each unit consisting of one share of our common stock and one half of one common stock purchase warrant. Each whole warrant will entitle its holder to purchase one share of our common stock at an exercise price of $2.00 per share at any time for 24 months after the closing of this offering. No units will be offered or sold in the United States. |
| Up to a total of 18,056,250 shares of our common stock will be issued or issuable in the offering, consisting of (i) 11,250,000 shares of common stock included in the units; (ii) up to 5,625,000 shares of common stock issuable upon exercise of the common stock purchase warrants included in the units; (iii) 787,500 shares of common stock included in the units issuable upon exercise of the unit options issued to the agent as compensation; and (iv) up to 393,750 shares of common stock issuable upon exercise of the common stock purchase warrants included with the units issuable under the agent’s unit options. If the volume weighted average trading price of our common stock on the TSX-V is equal to or exceeds $3.00 per share for 20 consecutive trading days after the date on which our common stock is first traded on the TSX-V, the Company shall have the right and option, exercisable at its sole discretion, to accelerate the expiration time of the warrants by providing written notice to each registered holder of warrants within five (5) business days and issuing a press release to the effect that the warrants will expire at 5:00 p.m. (Toronto time) on the date specified in such notice and press release, provided that such date shall not be less than 30 days following the date of such notice and press release. See “Plan of Distribution.” |
| Gross Proceeds |
$11,250,000, minimum. |
| Closing Date |
The offering will close as soon as practicable after being fully subscribed. Subscription funds will be held in trust by the agent until closing of the offering. No funds shall be released to us until such time as the minimum gross proceeds of $11,250,000 are received. If the minimum proceeds of $11,250,000 are not received on or before March 18, 2015, we will terminate the offering and the agent will promptly return all subscription funds to investors without interest or deduction. See “Plan of Distribution”. |
| Exercise of Company Put Rights |
In connection with a private placement of our Series B preferred stock to certain accredited investors, each purchaser of our Series B preferred stock executed a Put Agreement in our favor (each a “Put Agreement” and collectively the “Put Agreements”). Each Put Agreement gives us the right and option, exercisable in our sole discretion, to require each investor in our Series B preferred stock financing to purchase from us securities of the same type as those sold to investors in our initial public offering, at the same price as the securities sold in our initial public offering, up to a total purchase |
5
| amount per investor equal to the total purchase price for Series B preferred stock paid by such investor (our “Put Rights”) |
| In connection with this offering we have exercised our right to require the existing holders of our Series B preferred stock to purchase an aggregate of 2,700,000 units at a price of $1.00 per unit, for aggregate gross proceeds of $2,700,000. In accordance with the terms of the Put Agreements, following the exercise of our Put Rights all holders of our Series B Preferred Stock placed their purchase funds into an escrow account. Upon the closing of this offering, the escrowed funds will be released to us and we will issue the units to the purchasers. |
| The units issued pursuant to the exercise of our Put Rights will be issued separately from the units being offered hereby. The common stock and the warrants included with the units to be issued pursuant to our Put Rights have the same terms as the common stock and the warrants included with the units being offered hereby, except that the units issued pursuant to our Put Rights, the shares of common stock and warrants comprising such units, and the shares of common stock issuable upon exercise of such warrants will not be registered under the Securities Act. The issuance and sale of the units pursuant to the exercise of our Put Rights is subject to, and will be effective concurrently with, the closing of this offering. This offering is not contingent on the exercise of our Put Rights. |
| Series B Preferred Stock Conversion |
Pursuant to the terms of our Amended and Restated Certificate of Incorporation each share of our Series B preferred stock will be automatically converted into one share of our common stock upon the completion of this offering, except that the conversion rate applicable to the Series B preferred stock held by any Series B preferred stockholder who, following exercise of our Put Rights, fails to complete the purchase of units as required by the terms of the Put Agreement shall be adjusted downward so that such non-performing investor will be entitled upon such conversion to receive one-half of one share of common stock for each share of Series B preferred stock held by such non-performing investor. Following the exercise of our Put Rights, and in accordance with the Put Agreements, all holders of our Series B preferred stock placed their purchase funds into an escrow account. The escrowed funds will be released to us upon the closing of this offering without further action by the holders of our Series B preferred stock. Accordingly, no further action of the holders of our Series B preferred stock is required to comply with their obligations under the Put Agreements, and there will be no downward adjustment of the common shares issuable upon conversion of the Series B preferred stock. |
6
| Common stock to be outstanding after this offering |
32,265,343 shares* |
| Use of Proceeds |
The gross proceeds to us from the offering will be $11,250,000 and we estimate that the net proceeds to us from such amount, after payment of the agent’s commissions and offering-related expenses, would be approximately $10,212,500. Together with our estimated working capital of approximately $440,000 as of December 31, 2014, and gross proceeds of $2,700,000 from the issuance of units to certain existing investors pursuant to the exercise of our Put Rights described above, we will have funds available to us of approximately $13,352,500 after the offering. |
| We intend to use the funds available to us as follows: |
| • | Approximately $10,250,000 to fund research, development and pre-clinical testing activities, including costs associated with expansion of our internal scientific leadership and staff, lab facilities, equipment and supplies, and external contract research services; |
| • | Approximately $3,002,500 to fund general and administrative expenses; including increased legal, accounting, insurance and other administrative expenses associated with being a publicly traded company; and |
| • | Approximately $100,000 unallocated for general working capital. |
| For additional information see “Use of Proceeds.” |
| TSX Venture Exchange Listing |
The TSX-V has conditionally approved the listing of our common stock under the symbol “COB”. We do not currently intend to list our common stock on any exchange in the United States. The warrants will not be listed on any exchange. |
| TSX Venture Exchange Escrow Requirements |
In order to list the shares of our common stock on the TSX-V, the TSX-V requires that we comply with the escrow requirements imposed by National Policy 46-201 Escrow for Public Offerings. |
| * | Includes (i) the assumed conversion of all of our outstanding convertible preferred stock into an aggregate of 5,400,000 shares of our common stock immediately prior to completion of the offering and (ii) the issuance of an aggregate of 2,700,000 shares of our common stock included in the units to be issued pursuant to the exercise of our Put Rights in connection with this offering. Excludes (A) up to 2,609,811 shares of common stock issuable upon exercise of outstanding options granted under our 2011 Equity Incentive Plan; (B) up to 933,617 shares of common stock issuable upon exercise of outstanding common stock purchase warrants, (C) any of the shares issuable upon exercise of the common stock purchase warrants included in the units issued in this offering, (D) the shares of common stock included in the units issuable upon exercise of the unit options issued to the Agent as compensation in connection with the offering, (E) any of the shares issuable upon exercise of the common stock purchase warrants included in the units issuable upon exercise of the Agent’s unit options, (F) 1,350,000 shares issuable upon exercise of the common stock purchase warrants included in the units issued pursuant to the exercise of our Put Rights. |
7
| Pursuant to National Policy 46-201, all securities of our company held by directors, senior officers or persons holding more than 20% of the voting rights attached to the outstanding shares of our common stock immediately before and after the offering, and persons holding more than 10% of the voting rights attached to the outstanding shares of our common stock immediately before and after the offering and who have the right to elect one or more of our directors or senior officers will be subject to an escrow agreement prior to the closing of this offering. Subject to acceptance of our listing application, we expect to be listed as a “Tier 2” issuer on the TSX-V. As a Tier 2 issuer, these securities will be subject to the following release schedule: 10% of the securities are to be released upon the date of issuance of the final exchange bulletin respecting this offering and listing on the TSX-V and an additional 15% of the securities are to be released every 6 months thereafter. See “Shares Eligible for Future Sale-Escrowed Securities and Securities Subject to Contractual Restriction on Transfer”. |
| Manner of Offering |
The offering is occurring solely in Canada as conducted through Haywood Securities Inc., the agent, in accordance with the conditions of the Agency Agreement described under the heading “Plan of Distribution”. The offering is being conducted on a “commercially reasonable efforts, minimum offering” basis, which means that the agent will take all commercially reasonable steps to sell the units on our behalf, and must sell all 11,250,000 units if any are to be sold. |
| The Agent has been engaged by us solely to conduct sales in Canada, will limit its selling activity to residents of Canada and will not undertake any selling efforts in the United States or to U.S. Persons (as such term is defined in Rule 902(k) of Regulation S under the Securities Act of 1933, as amended). The Agent will conduct offers and sales of the units in Canada pursuant to a prospectus filed with securities commissions in each Province of Canada, other than Quebec. |
| A condition to closing of the offering is the effectiveness of a Registration Statement on Form S-1 registering the units and the securities underlying the units. Registration is being effected in the United States to permit the units and securities underlying the units to be issued in Canada without resale restrictions. |
| Agent Compensation |
As consideration for its services, Haywood Securities Inc. will receive the following: |
| (i) a cash commission equal to 4% of the gross proceeds from the sale of units in the offering to certain specified purchasers and 7% of the gross proceeds from the sale of units in the offering to all other purchasers; |
| (ii) unit options entitling the agent to purchase a number of units equal to 4% of the number of units sold under the offering to certain |
8
| specified purchasers and 7% of the number of units sold under the offering to all other purchasers for a period of 18 months from the closing date at a price of $1.00 per unit; and |
| (iii) a cash work fee of up to $30,000 payable in three equal monthly installments. |
| No commission or other form of compensation will be paid to any broker-dealer in the United States in connection with this offering. Haywood Securities Inc. will also be reimbursed for its reasonable fees and expenses, including reasonable fees, disbursements and applicable taxes of legal counsel to Haywood Securities Inc. See “Plan of Distribution.” |
9
Selected Summary Financial Data
The following tables present our summary financial data and should be read together with our financial statements and accompanying notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” appearing elsewhere in this prospectus. The summary financial data for the years ended December 31, 2013, 2012 and 2011 are derived from our audited annual financial statements included in the registration statement of which this prospectus forms a part. The unaudited summary financial data as of September 30, 2014 and for the nine month periods ended September 30, 2014 and 2013 have been derived from our unaudited interim financial statements included in the registration statement of which this prospectus forms a part, and include all adjustments, consisting of normal recurring adjustments, which in the opinion of management are necessary for a fair presentation of our financial position and results of operations for these periods.
| Selected Statement of Operations Information |
||||||||||||||||||||
| For the years ended December 31, | For the nine months ended September 30, |
|||||||||||||||||||
| 2013 | 2012 | 2011 | 2014 (Unaudited) |
2013 (Unaudited) |
||||||||||||||||
| Revenues |
$ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||
| Gross profit |
$ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||
| Total operating expenses |
$ | 869,005 | $ | 1,472,353 | $ | 292,229 | $ | 1,319,614 | $ | 635,911 | ||||||||||
| Net loss |
$ | (872,641 | ) | $ | (1,471,089 | ) | $ | (291,741 | ) | $ | (1,324,599 | ) | $ | (637,881 | ) | |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Basic and diluted net loss per share |
$ | (0.07 | ) | $ | (0.12 | ) | $ | (0.03 | ) | $ | (0.10 | ) | $ | (0.05 | ) | |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Weighted average common shares outstanding – basic and diluted |
12,915,343 | 12,094,629 | 10,129,681 | 12,915,343 | 12,915,343 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Selected Statement of Operations Information |
||||||||||||||||
| As of December 31, | As of September 30, 2014 (Unaudited) |
|||||||||||||||
| 2013 | 2012 | 2011 | ||||||||||||||
| Cash |
$ | 145,170 | $ | 878,094 | $ | 518,863 | $ | 1,818,843 | ||||||||
| Current assets |
$ | 286,489 | $ | 893,064 | $ | 520,463 | $ | 1,853,048 | ||||||||
| Total assets |
$ | 318,407 | $ | 900,185 | $ | 526,251 | $ | 2,103,896 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Current liabilities |
$ | 143,394 | $ | 74,136 | $ | 8,995 | $ | 399,568 | ||||||||
| Total liabilities |
$ | 348,007 | $ | 74,136 | $ | 8,995 | $ | 604,328 | ||||||||
| Total stockholders’ (deficiency) equity |
$ | (29,600 | ) | $ | 826,049 | $ | 517,256 | $ | 1,499,568 | |||||||
| Total liabilities and stockholders’ (deficiency) equity |
$ | 318,407 | $ | 900,185 | $ | 526,251 | $ | 2,103,896 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
10
An investment in our securities involves a high degree of risk. You should carefully consider the risks described below, together with all of the other information included in this prospectus, before making an investment decision. If any of the following risks actually occurs, our business, financial condition or results of operations could suffer. In that case, the market value of our securities could decline, and you may lose all or part of your investment.
Risks Related to Our Company
We have had a history of losses and no revenue, which raises substantial doubt about our ability to continue as a going concern.
Since our conversion to a Delaware corporation in September, 2009 through September 30, 2014, we have accumulated losses of $3,961,242. As of September 30, 2014, we had working capital of $1,453,480 and a stockholders’ equity of $1,499,568. We can offer no assurance that we will ever operate profitably or that we will generate positive cash flow in the future. To date, we have not generated any revenues from our operations. Our history of losses and no revenues raise substantial doubt about our ability to continue as a going concern. As a result, our management expects the business to continue to experience negative cash flow for the foreseeable future and cannot predict when, if ever, our business might become profitable. Until we can generate significant revenues, if ever, we expect to satisfy our future cash needs through equity or debt financing. We will need to raise additional funds, and such funds may not be available on commercially acceptable terms, if at all. If we are unable to raise funds on acceptable terms, we may not be able to execute our business plan, take advantage of future opportunities, or respond to competitive pressures or unanticipated requirements. This may seriously harm our business, financial condition and results of operations.
We are an early research stage biotechnology company and may never be able to successfully develop marketable products or generate any revenue. We have a very limited relevant operating history upon which an evaluation of our performance and prospects can be made. There is no assurance that our future operations will result in profits. If we cannot generate sufficient revenues, we may suspend or cease operations.
We are an early-stage company, have not generated any revenues to date and have no operating history. All of our MDPs are in the concept or research stage. Moreover, we cannot be certain that our research and development efforts will be successful or, if successful, that our MDPs or MDP analogs will ever be approved by the FDA. Even if approved, our products may not generate commercial revenues. We have no relevant operating history upon which an evaluation of our performance and prospects can be made. We are subject to all of the business risks associated with a new enterprise, including, but not limited to, risks of unforeseen capital requirements, failure of potential drug candidates either in research, pre-clinical testing or in clinical trials, failure to establish business relationships and competitive disadvantages against other companies. If we fail to become profitable, we may suspend or cease operations.
We will need additional funding and may be unable to raise additional capital when needed, which would force us to delay, reduce or eliminate our research and development activities.
Our operations to date have consumed substantial amounts of cash, and we expect our capital and operating expenditures to increase in the next few years. We believe that our existing capital resources and anticipated cash flow from planned operations, together with the anticipated proceeds from the issuance of units pursuant to the exercise of our Put Rights and of the net proceeds of this offering should be adequate to satisfy our cash requirements, including having at least $100,000 in unallocated funds, for at least the next 12 months. We will need to raise additional funding and we may be unable to raise additional needed capital on terms that are favorable to our company or at all. We may not be able to generate significant revenues for several years, if at all. Until we can generate significant revenues, if ever, we expect to satisfy our future cash needs through equity or
11
debt financing. We cannot be certain that additional funding will be available on acceptable terms, or at all. If adequate funds are not available, we may be required to delay, reduce the scope of, eliminate one or more of our research and development activities.
We may be unable to continue as a going concern in which case our securities will have little or no value.
Our independent registered public accountants have noted in their report concerning our annual financial statements for the fiscal year ended December 31, 2013 that we have incurred substantial losses since inception, which raises substantial doubt about our ability to continue as a going concern. In the event we are not able to continue operations you will likely suffer a complete loss of your investment in our securities.
Our independent registered public accountants have identified a material weakness in our internal control over financial reporting. In addition, because of our status as an emerging growth company, our independent registered public accountants are not required to provide an attestation report as to our internal control over financial reporting for the foreseeable future.
In connection with the contemporaneous audit of our consolidated financial statements for the years ended December 31, 2013, 2012 and 2011, our independent registered public accountants identified a material weakness in our internal control over financial reporting. A “material weakness” is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. The material weakness relates to our having one employee assigned to positions that involve processing financial information, resulting in a lack of segregation of duties so that all journal entries and account reconciliations are reviewed by someone other than the preparer, heightening the risk of error or fraud. If we are unable to remediate the material weakness, or other control deficiencies are identified, we may not be able to report our financial results accurately, prevent fraud or file our periodic reports as a public company in a timely manner.
As a public company, we will be required to maintain internal control over financial reporting and to report any material weaknesses in such internal control. In addition, beginning with our annual report on Form 10-K for the year ending December 31, 2015, we will be required to annually assess the effectiveness of our internal control over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act. We are in the process of designing, implementing, and testing the internal control over financial reporting required to comply with this obligation, which process is time consuming, costly, and complicated. Because of our limited resources and we may be unable remediate the identified material weakness in a timely manner, or additional control deficiencies may be identified. As a result, we may be unable to report our financial results accurately on a timely basis or help prevent fraud, which could cause our reported financial results to be materially misstated and result in the loss of investor confidence and cause the market price of our common stock to decline.
Whether or not our management concludes that our internal control over financial reporting is effective, our independent registered public accounting firm may issue a report that is qualified if it is not satisfied with our controls or the level at which our controls are documented, designed, operated or reviewed. However, our independent registered public accounting firm will not be required to attest formally to the effectiveness of our internal control over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act until the later of the year following our first annual report required to be filed with the SEC or the date we are no longer an “emerging growth company” as defined in the JOBS Act. We expect to be an “emerging growth company” for up to five years. Accordingly, you will not be able to depend on any attestation concerning our internal control over financial reporting from our independent registered public accountants for the foreseeable future.
Our ability to utilize our net operating loss carryforwards and certain other tax attributes may be limited.
Under Section 382 and related provisions of the Internal Revenue Code of 1986, as amended (the “Code”), if a corporation undergoes an “ownership change” (generally defined as a greater than 50% change (by value) in its equity ownership over a three year period), the corporation’s ability to use its pre-change net operating loss
12
carryforwards and other pre-change tax attributes to offset its post-change income may be limited. We may, upon completion of this offering, or in the future as a result of subsequent shifts in our stock ownership, experience, an “ownership change.” Thus, our ability to utilize carryforwards of our net operating losses and other tax attributes to reduce future tax liabilities may be substantially restricted. At this time, we have not completed a study to assess whether an ownership change under Section 382 of the Code may occur in the foreseeable future due to the costs and complexities associated with such a study. Further, U.S. tax laws limit the time during which these carryforwards may be applied against future taxes. Therefore, we may not be able to take full advantage of these carryforwards for federal or state tax purposes.
Risks Related to Our Business
Our short operating history may make it difficult for you to evaluate the success of our business to date and to assess our future viability.
We are an early-stage company. Our operations to date have been limited to organizing and staffing our company, business planning, raising capital, in-licensing intellectual property, identifying MDPs for further research and performing research on identified MDPs. All of our MDPs are still in the research phase. We have not yet demonstrated our ability to generate a pre-clinical or clinical drug candidate, initiate or successfully complete any clinical trials, including large-scale, pivotal clinical trials, obtain marketing approvals, manufacture a commercial scale medicine or arrange for a third party to do so on our behalf, or conduct sales and marketing activities necessary for successful commercialization. Typically, it takes 10-12 years to develop one new medicine from the time it is discovered to when it is available for treating patients and longer timeframes are not uncommon. Consequently, any predictions you make about our future success or viability may not be as accurate as they could be if we had a longer operating history.
In addition, as a new business, we may encounter unforeseen expenses, difficulties, complications, delays and other known and unknown factors. We will need to transition from a company with a research focus to a company capable of supporting development and commercialization activities. We may not be successful in such a transition.
We may not be successful in our efforts to identify or discover potential drug development candidates.
A key element of our strategy is to identify and test MDPs that play a role in cellular processes underlying our targeted disease indications. A significant portion of the research that we are conducting involves emerging scientific knowledge and drug discovery methods. Our drug discovery efforts may not be successful in identifying MDPs that are useful in treating disease. Our research programs may initially show promise in identifying potential drug development candidates, yet fail to yield candidates for pre-clinical and clinical development for a number of reasons, including:
| • | the research methodology used may not be successful in identifying appropriate potential drug development candidates; or |
| • | potential drug development candidates may, on further study, be shown not to be effective in humans, or to have unacceptable toxicities, harmful side effects, or other characteristics that indicate that they are unlikely to be medicines that will receive marketing approval and achieve market acceptance. |
Research programs to identify new product candidates require substantial technical, financial and human resources. We may choose to focus our efforts and resources on a potential product candidate that ultimately proves to be unsuccessful. If we are unable to identify suitable MDP analogs for pre-clinical and clinical development, we will not be able to obtain product revenues in future periods, which likely would result in significant harm to our financial position and adversely impact our stock price.
13
Our research and development plans will require substantial additional future funding which could impact our operational and financial condition. Without the required additional funds, we will likely cease operations.
It will take several years before we are able to develop potentially marketable products, if at all. Our research and development plans will require substantial additional capital to:
| • | conduct research, pre-clinical testing and human studies; |
| • | manufacture any future drug development candidate or product at pilot and commercial scale; and |
| • | establish and develop quality control, regulatory, and administrative capabilities to support these programs. |
Our future operating and capital needs will depend on many factors, including:
| • | the pace of scientific progress in our research programs and the magnitude of these programs; |
| • | the scope and results of pre-clinical testing and human studies; |
| • | the time and costs involved in obtaining regulatory approvals; |
| • | the time and costs involved in preparing, filing, prosecuting, securing, maintaining and enforcing intellectual property rights; |
| • | competing technological and market developments; |
| • | our ability to establish additional collaborations; |
| • | changes in any future collaborations; |
| • | the cost of manufacturing our drug products; and |
| • | the effectiveness of efforts to commercialize and market our products. |
We base our outlook regarding the need for funds on many uncertain variables. Such uncertainties include the success of our research and development initiatives, regulatory approvals, the timing of events outside our direct control such as negotiations with potential strategic partners and other factors. Any of these uncertain events can significantly change our cash requirements as they determine such one-time events as the receipt or payment of major milestones and other payments.
Additional funds will be required to support our operations and if we are unable to obtain them on favorable terms, we may be required to cease or reduce further research and development of our drug product programs, sell some or all of our intellectual property, merge with another entity or cease operations.
If we fail to demonstrate efficacy in our research and clinical trials our future business prospects, financial condition and operating results will be materially adversely affected.
The success of our research and development efforts will be greatly dependent upon our ability to demonstrate efficacy of MDP analogs in non-clinical studies, as well as in clinical trials. Non-clinical studies involve testing potential MDP-derived drugs in appropriate non-human disease models to demonstrate efficacy and safety. Regulatory agencies evaluate these data carefully before they will approve clinical testing in humans. If certain non-clinical data reveals potential safety issues or the results are inconsistent with an expectation of the potential drug’s efficacy in humans, the program may be discontinued or the regulatory agencies may require additional testing before allowing human clinical trials. This additional testing will increase program expenses and extend timelines. We may decide to suspend further testing on our potential drugs if, in the judgment of our management and advisors, the non-clinical test results do not support further development.
Moreover, success in research, pre-clinical testing and early clinical trials does not ensure that later clinical trials will be successful, and we cannot be sure that the results of later clinical trials will replicate the results of prior clinical trials and non-clinical testing. The clinical trial process may fail to demonstrate that our potential
14
drug candidates are safe for humans and effective for indicated uses. This failure would cause us to abandon a drug candidate and may delay development of other potential drug candidates. Any delay in, or termination of, our non-clinical testing or clinical trials will delay the filing of an investigational new drug application and new drug application with the Food and Drug Administration or the equivalent applications with pharmaceutical regulatory authorities outside the United States and, ultimately, our ability to commercialize our potential drugs and generate product revenues. In addition, we expect that our early clinical trials will involve small patient populations. Because of the small sample size, the results of these early clinical trials may not be indicative of future results.
Following successful non-clinical testing, potential drugs will need to be tested in a clinical development program to provide data on safety and efficacy prior to becoming eligible for product approval and licensure by regulatory agencies.
If any of our future potential drugs in clinical development become the subject of problems, our ability to sustain our development programs will become critically compromised. For example, efficacy or safety concerns may arise, whether or not justified, that could lead to the suspension or termination of our clinical programs. Examples of problems that could arise include, among others:
| • | efficacy or safety concerns with the potential drug candidates, even if not justified; |
| • | failure of agencies to approve a drug candidate and/or requiring additional clinical or non-clinical studies before prior to determining approvability; |
| • | manufacturing difficulties or concerns; |
| • | regulatory proceedings subjecting the potential drug candidates to potential recall; |
| • | publicity affecting doctor prescription or patient use of the potential drugs; |
| • | pressure from competitive products; or |
| • | introduction of more effective treatments. |
Each clinical phase is designed to test attributes of the drug and problems that might result in the termination of the entire clinical plan. These problems can be revealed at any time throughout the overall clinical program. The failure to demonstrate efficacy in our clinical trials would have a material adverse effect on our future business prospects, financial condition and operating results.
Even if we are able to develop our potential drugs, we may not be able to receive regulatory approval, or if approved, we may not be able to generate significant revenues or successfully commercialize our products, which will adversely affect our financial results and financial condition and we will have to delay or terminate some or all of our research and development plans which may force us to cease operations.
All of our potential drug candidates will require extensive additional research and development, including pre-clinical testing and clinical trials, as well as regulatory approvals, before we can market them. We cannot predict if or when any potential drug candidate we intend to develop will be approved for marketing. There are many reasons that we may fail in our efforts to develop our potential drug candidates. These include:
| • | the possibility that pre-clinical testing or clinical trials may show that our potential drugs are ineffective and/or cause harmful side effects or toxicities; |
| • | our potential drugs may prove to be too expensive to manufacture or administer to patients; |
| • | our potential drugs may fail to receive necessary regulatory approvals from the United States Food and Drug Administration or foreign regulatory authorities in a timely manner, or at all; |
| • | even if our potential drugs are approved, we may not be able to produce them in commercial quantities or at reasonable costs; |
| • | even if our potential drugs are approved, they may not achieve commercial acceptance; |
15
| • | regulatory or governmental authorities may apply restrictions to any of our potential drugs, which could adversely affect their commercial success; and |
| • | the proprietary rights of other parties may prevent us or our potential collaborative partners from marketing our potential drugs. |
If we fail to develop our potential drug candidates, our financial results and financial condition will be adversely affected, we will have to delay or terminate some or all of our research and development plans and may be forced to cease operations.
If we do not maintain the support of qualified scientific collaborators, our revenue, growth and profitability will likely be limited, which would have a material adverse effect on our business.
We will need to maintain our existing relationships with leading scientists and/or establish new relationships with scientific collaborators. We believe that such relationships are pivotal to establishing products using our technologies as a standard of care for various indications. There is no assurance that our founders, scientific advisors or research partners will continue to work with us or that we will be able to attract additional research partners. If we are not able to establish scientific relationships to assist in our research and development, we may not be able to successfully develop our potential drug candidates. If this happens, our business will be adversely affected.
We will seek to establish development and commercialization collaborations, and, if we are not able to establish them on commercially reasonable terms, we may have to alter our development and commercialization plans.
Our potential drug development programs and the potential commercialization of our drug candidates will require substantial additional cash to fund expenses. We may decide to collaborate with pharmaceutical or biotechnology companies in connection with the development or commercialization of our potential drug candidates.
We face significant competition in seeking appropriate collaborators. Whether we reach a definitive collaboration agreement will depend, among other things, upon our assessment of the collaborator’s resources and expertise, the terms and conditions of the proposed collaboration and the proposed collaborator’s evaluation of a number of factors. Those factors may include the design or results of clinical trials, the likelihood of approval by the FDA or similar regulatory authorities outside the United States, the potential market for the subject product candidate, the costs and complexities of manufacturing and delivering such product candidate to patients, the potential of competing products, the existence of uncertainty with respect to our ownership of technology, which can exist if there is a challenge to such ownership without regard to the merits of the challenge and industry and market conditions generally. The collaborator may also consider alternative product candidates or technologies for similar indications that may be available to collaborate on, and whether such alternative collaboration project could be more attractive than the one with us for our product candidate.
Collaborations are complex and time-consuming to negotiate and document. In addition, there have been a significant number of recent business combinations among large pharmaceutical companies that have resulted in a reduced number of potential future collaborators.
We may not be able to negotiate collaborations on a timely basis, on acceptable terms, or at all. If we are unable to do so, we may have to curtail the development of the product candidate for which we are seeking to collaborate, reduce or delay its development program or one or more of our other development programs, delay its potential commercialization or reduce the scope of any sales or marketing activities, or increase our expenditures and undertake development or commercialization activities at our own expense. If we elect to increase our expenditures to fund development or commercialization activities on our own, we may need to
16
obtain additional capital, which may not be available to us on acceptable terms or at all. If we do not have sufficient funds, we may not be able to further develop our product candidates or bring them to market and generate product revenue.
We expect to rely on third parties to conduct our clinical trials and some aspects of our research and pre-clinical testing. These third parties may not perform satisfactorily, including failing to meet deadlines for the completion of such trials, research or pre-clinical testing.
We currently rely on third parties to conduct some aspects of our research and expect to continue to rely on third parties to conduct additional aspects of our research and pre-clinical testing, as well as any future clinical trials. Any of these third parties may terminate their engagements with us at any time. If we need to enter into alternative arrangements, it would delay our product research and development activities.
Our reliance on these third parties for research and development activities will reduce our control over these activities but will not relieve us of our responsibilities. For example, we will remain responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocols for the trial. Moreover, the FDA requires us to comply with standards, commonly referred to as Good Clinical Practices, for conducting, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. We also are required to register ongoing clinical trials and post the results of completed clinical trials on a government-sponsored database, ClinicalTrials.gov, within certain timeframes. Failure to do so can result in fines, adverse publicity and civil and criminal sanctions.
Furthermore, these third parties may also have relationships with other entities, some of which may be our competitors. If these third parties do not successfully carry out their contractual duties, meet expected deadlines or conduct our clinical trials in accordance with regulatory requirements or our stated protocols, we will not be able to obtain, or may be delayed in obtaining, marketing approvals for our drug candidates and will not be able to, or may be delayed in our efforts to, successfully commercialize our medicines.
We also expect to rely on other third parties to store and distribute drug supplies for our clinical trials. Any performance failure on the part of our distributors could delay clinical development or marketing approval of our drug candidates or commercialization of our products, producing additional losses and depriving us of potential product revenue.
We may not be able to develop drug candidates, market or generate sales of our products to the extent anticipated. Our business may fail and investors could lose all of their investment in our Company.
Assuming that we are successful in developing our potential drug candidates and receiving regulatory clearances to market our potential products, our ability to successfully penetrate the market and generate sales of those products may be limited by a number of factors, including the following:
| • | if our competitors receive regulatory approvals for and begin marketing similar products in the United States, the European Union, Japan and other territories before we do, greater awareness of their products as compared to ours will cause our competitive position to suffer; |
| • | information from our competitors or the academic community indicating that current products or new products are more effective or offer compelling other benefits than our future products could impede our market penetration or decrease our future market share; and |
| • | the pricing and reimbursement environment for our future products, as well as pricing and reimbursement decisions by our competitors and by payers, may have an effect on our revenues. |
If any of these happened, our business could be adversely affected.
17
We contract with third parties for the manufacture of our peptide materials for research and expect to continue to do so for any future product candidate advanced to pre-clinical testing, clinical trials and commercialization. This reliance on third parties increases the risk that we will not have sufficient quantities of our research peptide materials, product candidates or medicines, or that such supply will not be available to us at an acceptable cost, which could delay, prevent or impair our research, development or commercialization efforts.
We do not have manufacturing facilities adequate to produce our research peptide materials or supplies of any future product candidate. We currently rely, and expect to continue to rely, on third-party manufacturers for the manufacture of our peptide materials, any future product candidates for pre-clinical and clinical testing, and for commercial supply of any of these product candidates for which we or future collaborators obtain marketing approval. We do not have long term supply agreements with any third-party manufacturers, and we purchase our research peptides on a purchase order basis.
We may be unable to establish any agreements with third-party manufacturers or to do so on acceptable terms. Even if we are able to establish agreements with third-party manufacturers, reliance on third-party manufacturers entails additional risks, including:
| • | reliance on the third party for regulatory compliance and quality assurance; |
| • | the possible breach of the manufacturing agreement by the third party; |
| • | the possible termination or nonrenewal of the agreement by the third party at a time that is costly or inconvenient for us; and |
| • | reliance on the third party for regulatory compliance, quality assurance, and safety and pharmacovigilance reporting. |
Third-party manufacturers may not be able to comply with current good manufacturing practices, or cGMP, regulations or similar regulatory requirements outside the United States. Our failure, or the failure of our third-party manufacturers, to comply with applicable regulations could result in sanctions being imposed on us, including fines, injunctions, civil penalties, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of product candidates or medicines, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect supplies of our medicines and harm our business and results of operations.
Any drug candidate that we may develop may compete with other drug candidates and products for access to manufacturing facilities. There are a limited number of manufacturers that operate under cGMP regulations and that might be capable of manufacturing for us.
Our current and anticipated future dependence upon others for the manufacture of our investigational materials or future product candidates or medicines may adversely affect our future profit margins and our ability to commercialize any medicines that receive marketing approval on a timely and competitive basis.
None of our potential drug candidates may reach the commercial market for a number of reasons and our business may fail.
Successful research and development of pharmaceutical products is high risk. Most development candidates fail to reach the market. Our success depends on the discovery of new drug candidates that we can commercialize. It is possible that our products may never reach the market for a number of reasons. They may be found ineffective or may cause harmful side-effects during non-clinical testing or clinical trials or fail to receive necessary regulatory approvals. We may find that certain products cannot be manufactured at a commercial scale and, therefore, they may not be economical to produce. Our potential products could also fail to achieve market acceptance or be precluded from commercialization by proprietary rights of third parties. Our licensed patents, patent applications, trademarks and other intellectual property may be challenged and this may delay or prohibit
18
us from effectively commercializing our products. Furthermore, we do not expect our potential drug candidates to be commercially available for a number of years, if at all. If none of our potential drug candidates reach the commercial market, our business will likely fail and investors will lose all of their investment in our Company. If this happens, our business will be adversely affected.
Any product candidate we are able to develop and commercialize would compete in the marketplace with existing therapies and new therapies that may become available in the future. These competitive therapies may be more effective, less costly, more easily administered, or offer other advantages over any product we seek to market.
There are numerous therapies currently marketed to treat diabetes, cancer, Alzheimer’s disease and other diseases for which our potential product candidates may be indicated. For example, if we develop an approved treatment for Type 2 Diabetes, it would compete with several classes of drugs for Type 2 Diabetes that are approved to improve glucose control. These include the insulin sensitizers pioglitazone (Actos) and rosiglitazone (Avandia), which are administered as oral once daily pills, and metformin, which is sometimes called an insulin sensitizer and is available as a generic once daily formulation. If we develop an approved treatment for Alzheimer’s disease it would compete with approved therapies such as donepezil (Aricept), galantamine (Razadyne), memantine (Namenda), rivastigmine (Exelon) and tacrine (Cognex). These therapies are varied in their design, therapeutic application and mechanism of action and may provide significant competition for any of our product candidates for which we obtain market approval. New products may also become available that provide efficacy, safety, convenience and other benefits that are not provided by currently marketed therapies. As a result, they may provide significant competition for any of our product candidates for which we obtain market approval. Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any products that we may develop. Our competitors also may obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market. In addition, our ability to compete may be affected in many cases by insurers or other third-party payors seeking to encourage the use of existing products which are generic or are otherwise less expensive to provide.
Our future success depends on key members of our scientific team and our ability to attract, retain and motivate qualified personnel.
We are highly dependent on our founders, Dr. Pinchas Cohen and Dr. Nir Barzilai, and the other principal members of our management and scientific teams. Drs. Cohen and Barzilai are members of our board of directors and provide certain scientific and research advisory services to us pursuant to consulting arrangements with each of them. Other members of our key management and scientific teams are employed “at will,” meaning we or they may terminate the employment relationship at any time. Our consultants and advisors, including our founders, may be employed by employers other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us. In addition, we rely on other consultants and advisors from time to time, including drug discovery and development advisors, to assist us in formulating our research and development strategy. Agreements with these advisors typically may be terminated by either party, for any reason, on relatively short notice. We do not maintain “key person” insurance for any of the key members of our team. The loss of the services of any of these persons could impede the achievement of our research, development and commercialization objectives.
Recruiting and retaining qualified scientific, clinical, and managerial personnel will also be critical to our success. We may not be able to attract and retain these personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We also experience competition for the hiring of scientific and clinical personnel from universities and research institutions.
19
We expect to expand our research, development and regulatory capabilities, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations.
We expect to experience significant growth in the number of our employees and the scope of our operations, particularly in the areas of research, drug development and regulatory affairs. To manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. Due to our limited financial resources and the limited experience of our management team in managing a company with such anticipated growth, we may not be able to effectively manage the expected expansion of our operations or recruit and train additional qualified personnel. Any inability to manage growth could delay the execution of our business plans or disrupt our operations.
The use of any of our products in clinical trials may expose us to liability claims, which may cost us significant amounts of money to defend against or pay out, causing our business to suffer.
The nature of our business exposes us to potential liability risks inherent in the testing, manufacturing and marketing of our products. We do not currently have any drug candidates in clinical trials, however, if any of our drug candidates enter into clinical trials or become marketed products, they could potentially harm people or allegedly harm people possibly subjecting us to costly and damaging product liability claims. Some of the patients who participate in clinical trials are already ill when they enter a trial or may intentionally or unintentionally fail to meet the exclusion criteria. The waivers we obtain may not be enforceable and may not protect us from liability or the costs of product liability litigation. Although we intend to obtain product liability insurance which we believe is adequate, we are subject to the risk that our insurance will not be sufficient to cover claims. The insurance costs along with the defense or payment of liabilities above the amount of coverage could cost us significant amounts of money and management distraction from other elements of the business, causing our business to suffer.
The patent positions of biopharmaceutical products are complex and uncertain and we may not be able to protect our patented or other intellectual property. If we cannot protect this property, we may be prevented from using it or our competitors may use it and our business could suffer significant harm. Also, the time and money we spend on acquiring and enforcing patents and other intellectual property will reduce the time and money we have available for our research and development, possibly resulting in a slow down or cessation of our research and development.
We are the exclusive licensee of patents and patent applications related to our MDPs and expect to own or license patents related to our potential drug candidates. However, neither patents nor patent applications ensure the protection of our intellectual property for a number of reasons, including the following:
| • | The United States Supreme Court recently rendered a decision in Molecular Pathology vs. Myriad Genetics, Inc., 133 S.Ct. 2107 (2013) (“Myriad”), in which the court held that naturally occurring DNA segments are products of nature and not patentable as compositions of matter. On March 4, 2014, the U.S. Patent and Trademark Office (“USPTO”) issued guidelines for examination of such claims that, among other things, extended the Myriad decision to any natural product. Since MDPs are natural products isolated from cells, the USPTO guidelines may affect allowability of some of our patent claims that are filed in the USPTO but are not yet issued. Further, while the USPTO guidelines are not binding on the courts, it is likely that as the law of subject matter eligibility continues to develop Myriad will be extended to natural products other than DNA. Thus, our issued U.S. patent claims directed to MDPs as compositions of matter may be vulnerable to challenge by competitors who seek to have our claims rendered invalid. While Myriad and the USPTO guidelines described above will affect our patents only in the United States, there is no certainty that similar laws or regulations will not be adopted in other jurisdictions. |
| • | Competitors may interfere with our patenting process in a variety of ways. Competitors may claim that they invented the claimed invention prior to us. Competitors may also claim that we are infringing their |
20
| patents and restrict our freedom to operate. Competitors may also contest our patents and patent applications, if issued, by showing in various patent offices that, among other reasons, the patented subject matter was not original, was not novel or was obvious. In litigation, a competitor could claim that our patents and patent applications are not valid or enforceable for a number of reasons. If a court agrees, we would lose some or all of our patent protection. |
| • | As a company, we have no meaningful experience with competitors interfering with our patents or patent applications. In order to enforce our intellectual property, we may even need to file a lawsuit against a competitor. Enforcing our intellectual property in a lawsuit can take significant time and money. We may not have the resources to enforce our intellectual property if a third party infringes an issued patent claim. Infringement lawsuits may require significant time and money resources. If we do not have such resources, the licensor is not obligated to help us enforce our patent rights. If the licensor does take action by filing a lawsuit claiming infringement, we will not be able to participate in the suit and therefore will not have control over the proceedings or the outcome of the suit. |
| • | Because of the time, money and effort involved in obtaining and enforcing patents, our management may spend less time and resources on developing potential drug candidates than they otherwise would, which could increase our operating expenses and delay product programs. |
| • | Our licensed patent applications directed to the composition and methods of using MOTS-c, our lead research peptide, and SHLP-6, which we consider as our primary research peptide for the potential treatment of cancer, have not yet been issued. There can be no assurance that these or our other licensed patent applications will result in the issuance of patents, and we cannot predict the breadth of claims that may be allowed in our currently pending patent applications or in patent applications we may file or license from others in the future. |
| • | Issuance of a patent may not provide much practical protection. If we receive a patent of narrow scope, then it may be easy for competitors to design products that do not infringe our patent(s). |
| • | We have limited ability to expand coverage of our licensed patent related to SHLP-2 and our licensed patent application related to SHLP-6 outside of the United States. The lack of patent protection in international jurisdictions may inhibit our ability to advance drug candidates derived from these MDPs in these markets. |
| • | If a court decides that the method of manufacture or use of any of our drug candidates, infringes on a third-party patent, we may have to pay substantial damages for infringement. |
| • | A court may prohibit us from making, selling or licensing a potential drug candidate unless the patent holder grants a license. A patent holder is not required to grant a license. If a license is available, we may have to pay substantial royalties or grant cross licenses to our patents, and the license terms may be unacceptable. |
| • | Redesigning our potential drug candidates so that they do not infringe on other patents may not be possible or could require substantial funds and time. |
It is also unclear whether our trade secrets are adequately protected. While we use reasonable efforts to protect our trade secrets, our employees or consultants may unintentionally or willfully disclose our information to competitors. Enforcing a claim that someone illegally obtained and is using our trade secrets is expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States are sometimes less willing to protect trade secrets. Our competitors may independently develop equivalent knowledge, methods and know-how. We may also support and collaborate in research conducted by government organizations, hospitals, universities or other educational institutions. These research partners may be unable or unwilling to grant us exclusive rights to technology or products derived from these collaborations prior to entering into the relationship.
If we do not obtain required intellectual property rights, we could encounter delays in our drug development efforts while we attempt to design around other patents or even be prohibited from developing,
21
manufacturing or selling potential drug candidates requiring these rights or licenses. There is also a risk that disputes may arise as to the rights to technology or potential drug candidates developed in collaboration with other parties.
Risks Related to this Offering
The offering price for our units may not be indicative of their fair value.
The offering price for our units was determined in the context of negotiations between us and the agent. Accordingly, the offering price may not be indicative of the true fair value of our company or the fair value of our units. We are making no representations that the offering price of our units under this prospectus bears any relationship to our assets, book value, net worth or any other recognized criteria of our value.
There is currently no trading market for our securities and an established trading market may not develop.
Our securities are not currently listed or quoted on any national securities exchange or national quotation system. We have received conditional approval for the listing of the shares of our common stock included in the units offered under this prospectus and the shares of our common stock issuable upon exercise of the warrants included in the units offered under this prospectus in Canada on the TSX-V. We do not currently intend to list our common stock on any exchange in the United States. We do not intend to list the warrants offered under this prospectus on any securities exchange. Listing of our common stock will be subject to fulfilling all of the requirements of the TSX-V. The TSX-V, or any other exchange or quotation system, may not permit our common stock to be listed and traded. Even if our common stock is accepted for listing on the TSX-V, the TSX-V has continuing listing requirements and we may not be able to comply with those requirements and maintain our listing.
If securities or industry analysts do not publish or cease publishing research or reports about us, our business or our market, or if they change their recommendations regarding our stock adversely, our stock price and trading volume could decline.
The trading market for our common stock will be influenced by the research and reports that industry or securities analysts may publish about us, our business, our market, or our competitors. If any of the analysts who may cover us change their recommendation regarding our stock adversely, or provide more favorable relative recommendations about our competitors, our stock price would likely decline. If any analysts who may cover us were to cease coverage or our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
Shares of our common stock eligible for future sale in the public marketplace may adversely affect the market price of our common stock.
The price of our common stock could decline if there are substantial sales of our common stock in the public stock market after this offering. Assuming the offering is fully subscribed, and after giving effect to the conversion of our Series B preferred stock into 5,400,000 shares of common stock and the issuance of 2,700,000 units pursuant to the exercise of our Put Rights, we anticipate that 32,265,343 shares of our common stock will be outstanding. All of the shares sold in this offering will be freely tradable without restriction or further registration under the Securities Act, except for any of those shares held by our “affiliates,” as that term is defined in Rule 144 under the Securities Act, whose sales would be subject to the volume and manner of sale limitations of Rule 144 described below. As of the date of this prospectus there are 12,915,343 shares of our common stock outstanding, 2,609,811 shares of our common stock reserved for issuance upon exercise of outstanding stock options, 5,400,000 shares of our common stock reserved for issuance upon conversion of our Series B preferred stock, 2,700,000 shares of our common stock reserved for issuance as part of the units to be issued pursuant to the exercise of our Put Rights, and 933,617 shares of our common stock issuable upon the exercise of our currently outstanding warrants (not including the warrants included in the units in this offering,
22
issuable to our agent in connection with this offering, or issuable pursuant to the exercise of our Put Rights), a substantial portion of which may be available for resale soon after the closing of this offering. For additional information, see “Shares Eligible for Future Sale.”
We have agreed with the holders of certain shares of our common stock to file, approximately 180 days after the completion of this offering, a registration statement covering the resale of shares of common stock held by such holders. We also intend to register for resale all shares of common stock issuable upon exercise of equity awards granted under our option plans. Once we register these shares, subject to any lock-up restrictions and resale restrictions imposed by the TSX-V, they can be freely sold in the public market. Due to these factors, sales of a substantial number of shares of our common stock in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of shares are able to or intend to sell shares, could reduce the market price of our common stock. For additional information, see “Dilution,” “Description of Capital Stock – Registration Rights” and “Shares Eligible for Future Sale – Canadian and TSX-V Resale Provisions.”
The market price of our common stock may be highly volatile and you may not be able to resell your shares at or above the initial public offering price.
Prior to this offering, there has been no public market for our securities. We have received conditional approval for listing on the TSX-V of the shares of our common stock included in the units offered under this prospectus and the shares of our common stock issuable upon exercise of the warrants included in the units offered under this prospectus. We do not currently intend to list our common stock on any exchange in the United States. An active trading market for our common stock may not develop following this offering. You may not be able to sell your shares quickly or at the market price if trading in our common stock is not active.
The market for our common stock will likely be characterized by significant price volatility when compared to more established issuers and we expect that it will continue to be so for the foreseeable future. The market price of our common stock is likely to be volatile for a number of reasons. First, our common stock is likely to be sporadically and/or thinly traded. As a consequence of this lack of liquidity, the trading of relatively small quantities of common stock by our stockholders may disproportionately influence the price of the common stock in either direction. The price of the common stock could, for example, decline precipitously if even a relatively small number of shares are sold on the market without commensurate demand, as compared to a market for shares of an established issuer which could better absorb those sales without adverse impact on its share price. Secondly, we are a speculative or “risky” investment due to our small amount of sales and lack of profits to date and uncertainty of future market acceptance for our current and potential products or services. As a consequence of this enhanced risk, more risk-adverse investors may, under the fear of losing all or most of their investment in the event of negative news or lack of progress, be more inclined to sell their shares on the market more quickly and at greater discounts than would be the case with the shares of an established issuer. We cannot make any predictions or projections as to what the prevailing market price for our common stock will be at any time or as to what effect the sale of common stock or the availability of common stock for sale at any time will have on the prevailing market price.
Purchasers of our units will experience immediate and substantial dilution as a result of their common stock being worth less on a net tangible book value basis than the amount they invested
The price that will be paid by investors in this offering for our units will be significantly higher than the net tangible book value per share of our common stock. Purchasers of our units will experience immediate and substantial dilution. In addition, all of our currently outstanding options, warrants and convertible preferred stock are exercisable for or convertible into shares of our common stock at prices that are at or below the expected purchase price that will be paid by investors in this offering. In connection with this offering, the warrants issued as part of the units sold in this offering will be exercisable for the purchase of 5,625,000 shares of our common stock for a period of 24 months from the closing date at a price of $2.00 per share; provided, that, if at any time the volume weighted average trading price of the shares of our common stock is equal to or exceeds $3.00 per
23
share for twenty (20) consecutive trading days after the date on which our common stock is first traded on the TSX-V, the Company shall have the right and option, exercisable at its sole discretion, to accelerate the expiration time of the warrants. The units issued as compensation to our agent will be exercisable for the purchase of up to 787,500 units for a period of 18 months from the closing date at a price of $1.00 per unit. There may be further dilution to investors following the exercise or conversion of our outstanding options or warrants upon effectiveness of this offering and to the extent that the warrants included in the units issued in the offering, pursuant to the exercise of our Put Rights, or to the agent in connection with this offering are exercised or converted. Accordingly, in the event we are liquidated, investors may not receive the full amount of their investment. For further information, see “Dilution.”
Our management owns a significant percentage of our outstanding common stock. If the ownership of our common stock continues to be highly concentrated in management, it may prevent other stockholders from influencing significant corporate decisions.
Our officers and directors currently own approximately 64.3% of the outstanding shares of our common stock, assuming the conversion of each share of our Series B preferred stock into one share of our common stock. After the sale of 11,250,000 units in the offering, and after giving effect to the conversion of our Series B preferred stock into 5,400,000 shares of common stock and the issuance of 2,700,000 units to certain existing investors pursuant to the exercise of our Put Rights (including 650,000 units anticipated to be issued pursuant to Put Agreements executed by our directors and officers), our executive officers and directors will own approximately 38.5% of the outstanding shares of our common stock after completion of the offering. Additionally, assuming the issuance of 650,000 units pursuant to Put Agreements with our directors and officers, our executive officers and directors will own options and warrants exercisable for an aggregate of 3,338,661 shares of our common stock, or 9.38% of our outstanding common stock after completion of the offering, assuming exercise of such options and warrants. As a result, our management will exercise significant control over matters requiring stockholder approval, including the election of our board of directors, the approval of mergers and other extraordinary transactions, as well as the terms of any of these transactions. This concentration of ownership could have the effect of delaying or preventing a change in our control or otherwise discouraging a potential acquirer from attempting to obtain control of us, which could in turn have an adverse effect on the fair market value of our company and our common stock. The interests of these and other of our existing stockholders may conflict with the interests of our other stockholders.
Management will have discretion as to the use of the proceeds from this offering, and may use the proceeds differently than explicitly described herein or not use the proceeds effectively.
While we intend to use the net proceeds of this offering and the funds available to us upon completion of the offering as described under the heading “Use of Proceeds”, circumstances may require us to adjust the application and allocation of such funds in order to address such changes or to take advantage of available opportunities. The success of our operations will be substantially dependent upon the discretion and judgment of our management with respect to the application and allocation of the funds available to us upon completion of this offering.
The requirements of being a public company may strain our resources, divert management’s attention and require us to disclose information that is helpful to competitors, make us more attractive to potential litigants and make it more difficult to attract and retain qualified personnel.
As a public company, we will be subject to the reporting requirements of the Securities Act, the Securities Exchange Act of 1934, as amended (Exchange Act), the Sarbanes-Oxley Act of 2002, as amended (Sarbanes-Oxley Act), the Dodd-Frank Wall Street Reform and Consumer Protection Act (Dodd-Frank Act), and applicable Canadian securities rules and regulations. Despite recent reforms made possible by the Jumpstart our Business Startups Act of 2012 (JOBS Act), compliance with these rules and regulations will nonetheless increase our legal and financial compliance costs, make some activities more difficult, time-consuming or costly and increase
24
demand on our systems and resources. The Exchange Act and applicable Canadian provincial securities legislation require, among other things, that we file annual, quarterly, and current reports with respect to our business and operating results.
Additionally, the Sarbanes-Oxley Act and the related rules and regulations of the SEC, as well as the rules and regulations of applicable Canadian securities regulators and the rules of the TSX-V (if our listing application is accepted), will require us to implement additional corporate governance practices and adhere to a variety of reporting requirements and complex accounting rules. Among other things, we will be subject to rules regarding the independence of the members of our board of directors and committees of the board and their experience in finance and accounting matters and certain of our executive officers will be required to provide certifications in connection with our quarterly and annual reports filed with the SEC and applicable Canadian securities regulators. The perceived increased personal risk associated with these rules may deter qualified individuals from accepting these positions. Accordingly, we may be unable to attract and retain qualified officers and directors. If we are unable to attract and retain qualified officers and directors, our business and our ability to obtain or maintain the listing of our shares of common stock on the TSX-V or another stock exchange could be adversely affected.
We are an “emerging growth company,” and we cannot be certain if the reduced reporting requirements applicable to emerging growth companies will make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We could be an emerging growth company for up to five years, although circumstances could cause us to lose that status earlier, including if we have more than $1.0 billion in annual revenue, the market value of our common stock held by non-affiliates exceeds $700 million as of any June 30 (the last day of our second fiscal quarter) before that time, or we issue more than $1.0 billion of non-convertible debt over a three-year period, in which case we would no longer be an emerging growth company as of the following December 31 (the last day of our fiscal year). We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
Under the JOBS Act, emerging growth companies can also delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies. Recent accounting standards that have been issued or proposed by the FASB or other standards-setting bodies that do not require adoption until a future date are not expected to have a material impact on our financial statements upon adoption.
Our common stock may be considered a “penny stock,” and thereby be subject to additional sale and trading regulations that may make it more difficult to sell.
Our common stock may be considered to be a “penny stock” if it does not qualify for one of the exemptions from the definition of “penny stock” under Rule 3a51-1 under the Exchange Act. Our common stock may be a “penny stock” unless one or more of the following conditions is met: (i) the stock trades at a price greater than $5.00 per share; (ii) it is traded on a national securities exchange in the United States; or (iii) we have net tangible assets greater than $2 million or average revenues of $6 million for the past three fiscal years.
25
The principal result or effect of being designated a “penny stock” is that U.S. securities broker-dealers participating in sales of our common stock will be subject to the “penny stock” regulations set forth in Rules 15g-2 through 15g-9 promulgated under the Exchange Act. For example, Rule 15g-2 requires broker-dealers dealing in penny stocks to provide potential investors with a document disclosing the risks of penny stocks and to obtain a manually signed and dated written receipt of the document at least two business days before effecting any transaction in a penny stock for the investor’s account. Moreover, Rule 15g-9 requires broker-dealers in penny stocks to approve the account of any investor for transactions in such stocks before selling any penny stock to that investor. This procedure requires the broker-dealer to (i) obtain from the investor information concerning his or her financial situation, investment experience and investment objectives; (ii) reasonably determine, based on that information, that transactions in penny stocks are suitable for the investor and that the investor has sufficient knowledge and experience as to be reasonably capable of evaluating the risks of penny stock transactions; (iii) provide the investor with a written statement setting forth the basis on which the broker-dealer made the determination in (ii) above; and (iv) receive a signed and dated copy of such statement from the investor, confirming that it accurately reflects the investor’s financial situation, investment experience and investment objectives. Compliance with these requirements may make it more difficult and time consuming for holders of our common stock to resell their shares to third parties or to otherwise dispose of them in the market or otherwise.
Prior to completion of the offering, we intend to adopt, and expect our existing stockholders will approve, for effectiveness following completion of the offering, a third amended and restated certificate of incorporation and amended and restated bylaws. The provisions of our third amended and restated certificate of incorporation, our amended and restated bylaws and Delaware law may discourage takeovers and business combinations that our stockholders might consider in their best interests.
Subject to compliance with applicable listing requirements of the TSX-V, we may determine to include in our third amended and restated certificate of incorporation and amended and restated bylaws adopted for effectiveness following completion of this offering provisions that may delay, defer, prevent or render more difficult a takeover attempt that our stockholders might consider in their best interests. For example, our third amended and restated certificate of incorporation includes a provision authorizing “blank check” preferred stock, which could be issued by our board of directors without stockholder approval and may contain voting, liquidation, dividend, and other rights superior to our common stock. Even in the absence of a takeover attempt, the existence of these provisions may adversely affect the market value of our common stock if they are viewed as discouraging takeover attempts in the future.
In addition, we may become subject to Section 203 of the Delaware General Corporation Law, which generally prohibits a Delaware corporation from engaging in any of a broad range of business combinations with an interested stockholder for a period of three years following the date on which the stockholder became an interested stockholder. This provision could have the effect of delaying or preventing a change of control, whether or not it is desired by or beneficial to our stockholders.
For additional information see “Description of Capital Stock.”
26
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus, including “Prospectus Summary,” “Risk Factors,” “Use of Proceeds,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business,” contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995 (collectively, “forward-looking statements”). Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events, and trends, the economy and other future conditions. In some cases you can identify these statements by forward-looking words such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “could,” “should,” “would,” “project,” “plan,” “expect,” “goal,” “seek,” “future,” “likely” or the negative or plural of these words or similar expressions. These forward-looking statements include, but are not limited to, the following:
| • | statements regarding anticipated outcomes of research, pre-clinical and clinical trials for our lead peptides and other MDPs; |
| • | expectations regarding the future market for any drug we may develop; |
| • | expectations regarding the growth of MDPs as a significant future class of therapeutic products; |
| • | statements regarding the anticipated therapeutic properties of drug development candidates derived from MDPs; |
| • | expectations regarding our ability to effectively protect our intellectual property; |
| • | statements concerning perceived competitive advantages and our ability to defend competitive advantages; |
| • | expectations regarding timeframes for identification and selection of an MDP drug candidate and completion of pre-clinical activities enabling submission of an investigational new drug application; |
| • | expectations regarding our ability to attract and retain qualified employees and key personnel; and |
| • | statements regarding the expected use of the proceeds of the offering. |
Because forward-looking statements relate to the future, they are subject to a number of risks, uncertainties and assumptions, which are difficult to predict and many of which are outside of our control, including those described in “Risk Factors.” Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this prospectus may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. Important factors that could cause our actual results to differ materially from those indicated in the forward-looking statements include, among other things, the following:
| • | our ability to successfully identify a suitable drug development candidate and conduct research, clinical and pre-clinical trials for our product candidates; |
| • | our ability to obtain required regulatory approvals to develop and market our product candidates; |
| • | our ability to raise additional capital on favorable terms; |
| • | our ability to execute our research and development plan on time and on budget; |
| • | our ability to obtain commercial partners; |
| • | our ability, whether alone or with commercial partners, to successfully develop and commercialize a product candidate; |
27
| • | our ability to identify and develop additional drug candidates; and |
| • | other risk factors included under “Risk Factors” in this prospectus. |
This list is not exhaustive of the factors that may affect our forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur. Any forward-looking statement made by us in this prospectus is based only on information currently available to us and speaks only as of the date on which it is made. Moreover, except as required by law, neither we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements. Except as required by applicable law, we undertake no obligation to update publicly any forward-looking statements for any reason after the date of this prospectus to conform these statements to actual results or to changes in our expectations.
28
USE OF MARKET AND INDUSTRY DATA
Unless otherwise indicated, information contained in this prospectus concerning our industry and the market in which we operate, including our general expectations, market opportunity and market development, is based on information from various sources, on assumptions that we have made that are based on those data and other similar sources and on our knowledge of the need for and markets associated with our potential products. These data involve a number of assumptions and limitations. While we believe the patient population, opportunity and market size information included in this prospectus is generally reliable, such information is inherently imprecise. In addition, projections, assumptions and estimates of our future performance or the future conditions in the industry in which we operate are necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described in “Risk Factors” and elsewhere in this prospectus. These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us.
29
The offering is subject to our receiving minimum gross proceeds of $11,250,000. We estimate that the net proceeds to us from such amount, after payment of the agent’s commissions and offering-related expenses, would be approximately $10,212,500. Together with our estimated working capital of approximately $440,000 as at December 31, 2014, and gross proceeds of $2,700,000 from the issuance of units pursuant to the exercise of our Put Rights, we will have funds available to us upon completion of the offering of approximately $13,352,500, calculated as follows:
| Funds Available |
||||||||
| Approximate working capital as of December 31, 2014 |
$ | 440,000 | ||||||
| Proceeds from issuance of units pursuant to the exercise of our Put Rights |
$ | 2,700,000 | ||||||
| Gross proceeds of the offering |
$ | 11,250,000 | ||||||
| Less: agent’s commission |
$ | (787,500 | ) | |||||
| Less: offering-related expenses |
$ | (250,000 | ) | |||||
|
|
|
|||||||
| Net proceeds of the offering |
$ | 10,212,500 | ||||||
|
|
|
|||||||
| Total |
$ | 13,352,500 | ||||||
|
|
|
|||||||
The principal purposes of this offering are to create a public market for our common stock, facilitate our future access to the public equity markets, increase awareness of our company and obtain additional capital to advance our research programs and the growth of our company.
We intend to use the funds available to us as follows:
| • | Approximately $10,250,000 to fund research, development and pre-clinical testing activities, including costs associated with expansion of our internal scientific leadership and staff, lab facilities, equipment and supplies, and external contract research services; |
| • | Approximately $3,002,500 to fund general and administrative expenses; including increased legal, accounting, insurance and other administrative expenses associated with being a publicly traded company; and |
| • | Approximately $100,000 unallocated for general working capital. |
The business objectives anticipated to be achieved with the funds available to us upon completion of the offering relate to:
| • | Identification, evaluation, optimization and selection of an MDP drug candidate; and |
| • | Completion of pre-clinical studies and other activities related to the filing and clearance of an Investigational New Drug (IND) application with the U.S. Food and Drug Administration to allow subsequent clinical trials of the selected candidate. |
Selection of an MDP drug candidate for IND-enabling activities includes the evaluation of our existing MDPs, and any newly discovered MDPs, in efficacy models, the prioritization of potential lead molecules, and the optimization of leads through synthesis of analogs and iterative evaluation of stability, pharmacokinetics, and efficacy in vitro and in vivo. We estimate that it will cost approximately $5,800,000 and require 12 to 18 months to complete activities leading to selection of a potential MDP drug candidate. Once candidate selection is completed, IND-enabling activities with respect to the selected candidate include pre-clinical studies related to candidate toxicology, safety pharmacology, genetic toxicity, pharmacokinetics, and manufacturing of drug substance and formulation in accordance with current good manufacturing practices. We estimate that these activities can be completed 12 to 18 months following candidate selection and will cost approximately $3,500,000.
The actual costs, timing and amount of funds required to complete the foregoing objectives cannot be determined precisely at this time, and may vary significantly depending on a number of factors, including the outcomes of our internal and external research activities including our MDP research and drug candidate
30
selection activities, the results of pre-clinical studies conducted on any MDP drug candidate we select, difficulties or unanticipated expenses associated with formulating or manufacturing pre-clinical supplies of candidate drug substance and the actual expenses of operating our business. The activities and costs described above do not include the initiation of clinical studies. We intend to spend the funds available as stated above; however, there may be circumstances where a reallocation of funds would be necessary. The actual use of the proceeds available to us after this offering will vary depending upon our operating and capital needs from time to time and will be subject to the discretion of management. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Our Research Programs” and “Risk Factors” for further information.
We experienced negative net cash flows from operating activities in the fiscal years ended December 31, 2013, 2012 and 2011 and in the nine months ended September 30, 2014 and 2013. We believe that our available cash, including the net proceeds of the sale, immediately prior to the closing of the offering, of our common stock pursuant to the exercise of our Put Rights, together with the proceeds of the offering will be sufficient to meet our cash needs for working capital and operating expenses for at least the next 12 months.
In addition to the proceeds to be received by us on the closing of the offering, we may receive up to an additional $11,250,000 from the exercise of the warrants issued as part of the units, and up to an additional $2,700,000 from the exercise of the warrants comprising a part of the units issued to existing investors pursuant to the exercise of our Put Rights. There can be no assurance that such warrants will be exercised. We expect to use the proceeds from the exercise of the warrants issued as part of the units, if any, for general working capital and operations.
We have never declared or paid, and do not anticipate declaring or paying in the foreseeable future, any cash dividends on our capital stock. Any future determination as to the declaration and payment of dividends, if any, will be at the discretion of our board of directors, subject to applicable laws and will depend on then existing conditions, including our financial condition, operating results, applicable TSX-V policies, contractual restrictions, capital requirements, business prospects, and other factors our board of directors may deem relevant.
31
The following table sets forth our cash and our capitalization as of September 30, 2014:
| • | on an actual basis; |
| • | on a pro forma basis after giving effect to (i) the automatic conversion, pursuant to the terms of our Second Amended and Restated Certificate of Incorporation, immediately upon completion of the offering of 5,400,000 shares of our outstanding convertible preferred stock into an aggregate of 5,400,000 shares of our common stock and (ii) sale and issuance of 2,700,000 units to certain existing investors pursuant to the exercise of our Put Rights, the purchase funds for which have been placed in escrow by such existing investors pending closing of the offering; and |
| • | on a pro forma as adjusted basis assuming the events described above and the sale in this offering of 11,250,000 units at the offering price of $1.00 per unit, resulting in net proceeds of $10,212,500, after deducting estimated agents’ commissions and offering expenses of $1,037,500. |
You should read this table together with our financial statements and related notes, “Selected Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” each included elsewhere in this prospectus.
| September 30, 2014 | ||||||||||||||||||||
| Actual (Unaudited) |
Adjustments | Pro Forma | Pro Forma Adjustments |
Pro Forma as Adjusted |
||||||||||||||||
| Cash and cash equivalents |
1,818,843 | 2,700,000 | 4,518,843 | 10,212,500 | 14,731,343 | |||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total long-term obligations |
204,760 | — | 204,760 | 204,760 | ||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Stockholders’ Equity: |
||||||||||||||||||||
| Preferred stock |
5,400 | (5,400 | ) | — | — | |||||||||||||||
| Common stock |
12,915 | 8,100 | 21,015 | 11,250 | 32,265 | |||||||||||||||
| Additional paid-in capital |
5,442,495 | 2,697,300 | 8,139,795 | 10,201,250 | 18,341,045 | |||||||||||||||
| Accumulated deficit |
(3,961,242 | ) | — | (3,961,242 | ) | — | (3,961,242 | ) | ||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total stockholders’ equity |
1,499,568 | 4,199,568 | 14,412,068 | |||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total capitalization |
1,704,328 | 4,404,328 | 14,616,828 | |||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
The number of shares of common stock to be outstanding immediately after this offering is based on the number of shares outstanding as of September 30, 2014, excluding a total of up to 11,699,678 shares of our common stock issuable upon exercise of the following securities:
| • | up to 2,609,811 shares issuable upon exercise of options granted under our 2011 Equity Incentive Plan outstanding as of the date of this prospectus; |
| • | up to 20,946 shares issuable upon exercise of outstanding stock purchase warrants, having an exercise price of $0.50 per share; |
| • | up to 15,596 shares issuable upon exercise of an outstanding stock purchase warrant, having an exercise price of $0.99 per share; |
| • | up to 897,075 shares issuable upon exercise of outstanding stock purchase warrants, having an exercise price of $0.26 per share; |
| • | up to 1,350,000 shares issuable upon exercise of the warrants to be issued as part of the units issued upon exercise of our Put Rights, having an exercise price of $2.00 per share; |
| • | up to 5,625,000 shares of common stock issuable upon exercise of the warrants included in the units issued in this offering; and |
32
| • | up to 787,500 shares issuable upon exercise of the unit options to be issued as compensation to the agent and up to 393,750 shares issuable upon exercise of the warrants included as part of the units issuable to the agent under the unit options. |
Pursuant to the terms of our Second Amended and Restated Certificate of Incorporation each share of our Series B preferred stock will be automatically converted to one share of our common stock upon the completion of this offering; except, that the conversion rate applicable to the Series B preferred stock held by any Series B preferred stockholder who fails, following exercise of our Put Rights, to purchase our securities as required by terms of the Put Agreement shall be adjusted downward so that such non-compliant investor will be entitled upon such conversion to receive one-half of one share of common stock for each share of Series B preferred stock held by such non-performing investor. Following the exercise of our Put Rights, and in accordance with the Put Agreements, all holders of our Series B preferred stock have placed their purchase funds into an escrow account. The escrowed funds will be released to us upon the closing of this offering without further action by the holders of our Series B preferred stock. Accordingly, no further action of the holders of our Series B preferred stock is required to comply with their obligations under the Put Agreements, and there will be no downward adjustment of the common shares issuable upon conversion of the Series B preferred stock.
33
If you invest in our units in this offering, your ownership interest will be diluted to the extent of the difference between the initial public offering price per unit and the pro forma as adjusted net tangible book value per share of our common stock after this offering. The pro forma net tangible book value of our common stock as of September 30, 2014 was $4,199,568, or $0.20 per share, based on the number of common shares outstanding as of September 30, 2014, assuming (i) the conversion of our convertible preferred stock outstanding on September 30, 2014 into 5,400,000 shares of common stock and (ii) sale and issuance pursuant to the exercise of our Put Rights of an aggregate of 2,700,000 shares of our common stock.
The following table sets forth our pro forma as adjusted net tangible book value as of September 30, 2014, assuming the sale in this offering of 11,250,000 units at the offering price of $1.00 per unit, after deducting estimated agents’ commissions and offering expenses of $1,037,500.
| Number of units sold in the offering |
11,250,000 | |||||||
| Offering price per unit |
$ | 1.00 | ||||||
| Pro forma net tangible book value per share as of September 30, 2014 |
$ | 0.20 | ||||||
| Increase in net tangible book value per share attributable to investors participating in this offering, after offering costs |
0.25 | |||||||
|
|
|
|||||||
| Pro forma as adjusted net tangible book value per share after this offering |
0.45 | |||||||
|
|
|
|||||||
| Pro forma dilution per share to investors participating in this offering |
$ | 0.55 | ||||||
|
|
|
The following table summarizes, on a pro forma as adjusted basis as of September 30, 2014, after giving effect to the conversion of all outstanding shares of convertible preferred stock, and the sale and issuance of shares of common stock included in the units issued pursuant to the exercise of our Put Rights, the differences between the number of shares of common stock purchased from us, the total consideration, and the average price per share paid by existing stockholders and by investors participating in this offering, assuming the sale in this offering of 11,250,000 units at the offering price of $1.00 per unit, after deducting estimated agents’ commissions and offering expenses of $1,037,500.
| Shares Purchased | Total Consideration | Average Price per Share |
||||||||||||||||||
| Number | Percent (%) | Amount | Percent (%) | |||||||||||||||||
| Existing stockholders before this offering |
21,015,343 | 65 | % | $ | 5,403,447 | 35 | % | $ | 0.26 | |||||||||||
| Investors participating in this offering |
11,250,000 | 35 | % | $ | 10,212,500 | 65 | % | $ | 0.91 | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total |
32,265,343 | 100 | % | $ | 15,615,947 | 100 | % | $ | 0.48 | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
The share data in the table above is based upon the number of shares of our common stock outstanding as of September 30, 2014 assuming (i) the conversion of our outstanding convertible preferred stock into 5,400,000 shares of common stock and (ii) sale and issuance pursuant to the exercise of our Put Rights of an aggregate of 2,700,000 shares of our common stock. This excludes the following securities outstanding as of September 30, 2014:
| • | up to 1,225,219 shares issuable upon exercise of outstanding options granted under our 2011 Equity Incentive Plan, with a weighted-average exercise price of $0.23 per share; |
| • | up to 20,946 shares issuable upon exercise of outstanding stock purchase warrants, having an exercise price of $0.50 per share; |
| • | up to 15,596 shares issuable upon exercise of an outstanding stock purchase warrant, having an exercise price of $0.99 per share; |
34
| • | up to 897,075 shares issuable upon exercise of outstanding stock purchase warrants, having an exercise price of $0.26 per share; and |
| • | 1,025,822 shares of our common stock reserved for future issuance under our 2011 Equity Incentive Plan as of September 30, 2014. |
To the extent that outstanding options or warrants are exercised you will experience further dilution. If all of our exercisable options and the warrants were exercised, our pro forma net tangible book value as of September, 2014 would have been $4,561,176, or $0.20 per share, and the pro forma, as adjusted net tangible book value after this offering would be $15,561,176, or $0.45 per share, causing dilution to new investors of $0.55 per share.
In addition, to the extent we choose to raise additional capital through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
35
You should read the following selected financial and other data below in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the financial statements, related notes, and other financial information included in this prospectus. The selected financial data in this section are not intended to replace the financial statements and are qualified in their entirety by the financial statements and related notes included elsewhere in this prospectus.
The summary financial data for the years ended December 31, 2013, 2012 and 2011 are derived from our audited annual financial statements, which are included elsewhere in this prospectus. The unaudited summary financial data as of September 30, 2014 and for the nine months ended September 30, 2014 and 2013 have been derived from our unaudited interim financial statements, which are included elsewhere in this prospectus, and include all adjustments, consisting of normal recurring adjustments, which in the opinion of management are necessary for a fair presentation of our financial position and results of operations for these periods.
| Selected Statement of Operations Information |
||||||||||||||||||||
| For the years ended December 31, | For the nine months ended September 30, |
|||||||||||||||||||
| 2013 | 2012 | 2011 | 2014 (Unaudited) |
2013 (Unaudited) |
||||||||||||||||
| Revenues |
$ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||
| Gross profit |
$ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||
| Total operating expenses |
$ | 869,005 | $ | 1,472,353 | $ | 292,229 | $ | 1,319,614 | $ | 635,911 | ||||||||||
| Net loss |
$ | (872,641 | ) | $ | (1,471,089 | ) | $ | (291,741 | ) | $ | (1,324,599 | ) | $ | (637,881 | ) | |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Basic and diluted net loss per share |
$ | (0.07 | ) | $ | (0.12 | ) | $ | (0.03 | ) | $ | (0.10 | ) | $ | (0.05 | ) | |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Weighted average common shares outstanding – basic and diluted |
12,915,343 | 12,094,629 | 10,129,681 | 12,915,343 | 12,915,343 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Selected Balance Sheet Information |
||||||||||||||||
| As of December 31, | As of September 30, |
|||||||||||||||
| 2013 | 2012 | 2011 | 2014 (unaudited) |
|||||||||||||
| Cash |
$ | 145,170 | $ | 878,094 | $ | 518,863 | $ | 1,818,843 | ||||||||
| Current assets |
$ | 286,489 | $ | 893,064 | $ | 520,463 | $ | 1,853,048 | ||||||||
| Total assets |
$ | 318,407 | $ | 900,185 | $ | 526,251 | $ | 2,103,896 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Current liabilities |
$ | 143,394 | $ | 74,136 | $ | 8,995 | $ | 399,568 | ||||||||
| Total liabilities |
$ | 348,007 | $ | 74,136 | $ | 8,995 | $ | 604,328 | ||||||||
| Total stockholders’ (deficiency) equity |
$ | (29,600 | ) | $ | 826,049 | $ | 517,256 | $ | 1,499,568 | |||||||
| Total liabilities and stockholders’ (deficiency) equity |
$ | 318,407 | $ | 900,185 | $ | 526,251 | $ | 2,103,896 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
36
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of financial condition and results of operations in conjunction with our financial statements and related notes included elsewhere in this prospectus. This discussion contains forward-looking statements based upon current expectations that involve risks and uncertainties. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of various factors, including those set forth under “Risk Factors” or in other parts of this prospectus.
Overview
We are a research stage biotechnology company committed to applying our scientific leadership in the biology of Mitochondrial-Derived Peptides, or MDPs, to extend the healthy lifespan and transform the lives of patients with major diseases.
Our founders and co-founders are widely considered to be scientific experts and thought leaders at the intersection of cellular and mitochondrial genetics and biology, the biology of aging, metabolism, and drug discovery and development. Their multi-disciplinary expertise and their investigations into age-related diseases has enabled and focused our research efforts on the mitochondrial genome.
MDPs represent a diverse and largely unexplored collection of peptides which we believe has the potential to lead to novel therapeutics for a number of diseases with significant unmet medical needs. We believe that Cohbar is a first mover in exploring the mitochondrial genome to identify MDPs with potential to be developed into transformative medicines, and that the depth of our scientific expertise, together with our intellectual property portfolio, will enable us to sustain this competitive advantage. By augmenting our scientific leadership and MDP discoveries with drug discovery and development expertise and capabilities, we believe we can identify and develop MDP-based therapeutic candidates that harness MDP cell-signaling mechanisms and unlock the therapeutic potential of this collection of peptides.
Our operations to date have been focused on organizing and staffing our company, business planning, raising capital and research on our MDPs. Our research efforts have focused on discovering and evaluating our MDPs for potential development as drug candidates. We seek to identify and advance research on MDPs with superior potential for yielding a drug candidate, and ultimately a drug, for which we have a strong intellectual property position.
Since our formation in 2007, we have in-licensed key intellectual property from our founders’ affiliated academic institutions, developed methods for identifying new MDPs, studied various MDPs in both in vitro and in vivo models and identified a number of MDPs with potential to lead to drug candidates for treatment of diabetes, cancer, Alzheimer’s disease, atherosclerosis and other diseases. Based on our evaluation of MDPs currently in our research pipeline we are actively engaged in research of four MDPs for potential advancement into drug candidate programs.
We are the exclusive licensee from the Regents of the University of California and the Albert Einstein College of Medicine to four issued U.S. patents and four U.S. and international patent applications. Our licensed patents and patent applications are directed to compositions comprising MDPs and MDP analogs and methods of their use in the treatment of indicated diseases. See “Business – Patents and Other Intellectual Property”.
To date, we have financed our operations primarily through private placements of our preferred stock and, to a lesser extent, from grants from research foundations. Since our inception through September 30, 2014 our operations have been funded with an aggregate of approximately $5.7 million, of which approximately $0.2 million was from a grant funding organizations and approximately $5.5 million was from the issuance of preferred stock.
37
Since inception, we have incurred significant operating losses. Our net losses were $1,324,599, $872,641 and $1,471,089 for the nine months ended September 30, 2014 and for the years ended December 31, 2013 and 2012, respectively. As of September 30, 2014, we had an accumulated deficit of $3,961,242. We expect to continue to incur significant expenses and operating losses over the next several years. Our net losses may fluctuate significantly from quarter to quarter and from year to year. We anticipate that our expenses will increase significantly when we commence pre-clinical development activities for any of our research peptides, continue research and discovery efforts on these and other MDPs, expand and protect our intellectual property portfolio, and hire additional development and scientific personnel. In addition, upon the closing of this offering we expect to incur additional costs associated with operating as a public company.
Financial Operations Review
Revenue
To date, we have not generated any revenue from product sales and do not expect to generate any revenue from the sale of products in the near future. In the future, we will seek to generate revenue from product sales, either directly or under any future licensing, development or similar relationship with a strategic partner.
Research and development expenses
Research and development expenses consist primarily of costs incurred for our research activities, including our drug discovery efforts, and the development of our product candidates, which include:
| • | employee-related expenses including salaries, benefits, and stock-based compensation expense; |
| • | expenses incurred under agreements with third parties, including contract research organizations, or CROs, that conduct research and development and pre-clinical activities on our behalf and the cost of consultants; |
| • | the cost of laboratory equipment, supplies and manufacturing MDP test materials; and |
| • | depreciation and other personnel-related costs associated with research and product development. |
We expense all research and development expenses as incurred.
Our Research Programs
Our research programs include activities related to discovery of MDPs, investigational research to evaluate the therapeutic potential of certain discovered MDPs and engineering analogs of certain discovered MDPs to improve their characteristics as potential drug development candidates. Depending on factors of capability, cost, efficiency and intellectual property rights we conduct our research programs independently at our laboratory facility, pursuant to contractual arrangements with CROs or under collaborative arrangements with academic institutions.
The success of our research programs and the timing of those programs and the possible development of a research peptide into a drug candidate is highly uncertain. As such, at this time, we cannot reasonably estimate or know the nature, timing and estimated costs of the efforts that will be necessary to complete research and development of a commercial drug. We are also unable to predict when, if ever, we will receive material net cash inflows from our operations. This is due to the numerous risks and uncertainties associated with developing medicines, including the uncertainty of:
| • | establishing an appropriate safety profile with toxicology studies; |
| • | successful enrollment in, and completion of clinical trials; |
| • | receipt of marketing approvals from applicable regulatory authorities; |
38
| • | establishing commercial manufacturing capabilities or making arrangements with third-party manufacturers; |
| • | obtaining and enforcing patent and trade secret protection for our product candidates; |
| • | launching commercial sales of the products, if and when approved, whether alone or in collaboration with others; and |
| • | a continued acceptable safety profile of the products following approval. |
A change in the outcome of any of these variables with respect to the development of any of our product candidates would significantly change the costs and timing associated with the development of that product candidate.
Research and development activities are central to our business model. Our drug target candidates are in early stages of investigational research. Candidates in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical trials. We expect research and development costs to increase significantly for the foreseeable future as our product candidate development programs progress. However, we do not believe that it is possible at this time to accurately project total program-specific expenses through commercialization. There are numerous factors associated with the successful commercialization of any of our product candidates, including future trial design and various regulatory requirements, many of which cannot be determined with accuracy at this time based on our stage of development. Additionally, future commercial and regulatory factors beyond our control will impact our clinical development programs and plans.
General and administrative expenses
General and administrative expenses consist primarily of salaries and other related costs, including stock-based compensation, for personnel in executive, finance and administrative functions. Other significant costs include facility costs not otherwise included in research and development expenses, legal fees relating to patent and corporate matters and fees for accounting and consulting services. We anticipate that our general and administrative expenses will increase in the future to support continued research and development activities, potential commercialization of our product candidates and increased costs of operating as a public company. These increases will likely include increased costs related to the hiring of additional personnel and fees to outside consultants, lawyers and accountants, among other expenses. Additionally, we anticipate increased costs associated with being a public company including expenses related to services associated with maintaining compliance with exchange listing and Securities and Exchange Commission requirements, insurance, and investor relations costs.
Results of Operations
Comparison of Fiscal Years Ended December 31, 2013 and 2012
Operating Expenses
Research and development expenses were $478,256 in the year ended December 31, 2013 compared to $854,292 in the prior year, a $376,036 decrease, or 44%. The decrease in research and development expenses in the year ended December 31, 2013, was primarily due to a $203,330 decrease in lab services and supplies due to lower overall usage in 2013 as compared to 2012, a $123,950 decrease in consultants costs due to the lower compensation levels in the current year period as compared to the prior year and lower salary and benefit costs of $78,979 due to a lower headcount of 2 employees in 2013 as compared to 2012, partially offset by a $49,368 increase in spending related to the grant from the Alzheimer’s Drug Discovery Foundation. We expect our research and development expenses will increase in the near term as we continue to increase our infrastructure and scientific research efforts.
39
General and administrative expenses were $390,749 in the year ended December 31, 2013 compared to $618,061 in the prior year, a $227,312 decrease, or 37%. The decrease in general and administrative expenses in the year ended December 31, 2013, was primarily due to a $157,087 decrease in compensation costs due to the decrease in salary and benefit costs in 2013 as compared to the prior year period and a $58,494 decrease in exclusive licensing fees in the current year as compared to the prior year, as 2012 included payment of initial licensing fees. We expect that our general and administrative expenses will increase in the near term as we incur additional expenses associated with a public offering and being a publicly-traded company.
Comparison of Fiscal Years Ended December 31, 2012 and 2011
Operating Expenses
Research and development expenses were $854,292 in the fiscal year ended December 31, 2012 compared to $109,301 in the prior fiscal year, a $744,991 increase. The increase in research and development expenses in the year ended December 31, 2012, was due to a $332,628 increase in lab supplies and services related to the increase in our scientific research efforts, a $241,400 increase in salary and benefit costs related to the increase in headcount of 4 employees, and the increase in consultants and Scientific Advisory Board member fees of $171,000 as we increased our utilization of those services.
General and administrative expenses were $618,061 in the year ended December 31, 2012 compared to $182,928 in the prior year, a $435,133 increase. The increase in general and administrative expenses in the year ended December 31, 2012, was due primarily to the increase in salary and benefit costs of $272,031, as 2012 included a full year of costs as compared to a partial year recognized in 2011, a $63,702 increase in exclusive licensing fees in the current year as compared to the prior year, as 2012 included the initial licensing fees and a $42,670 increase in legal costs due to increase use.
Comparison of Fiscal Years Ended December 31, 2011 and 2010
Operating Expenses
Research and development expenses were $109,301 in the year ended December 31, 2011 compared to $0 in the prior year. The increase in research and development expenses in the year ended December 31, 2011was primarily due to the increase in business activities.
General and administrative expenses were $182,928 in the year ended December 31, 2011 compared to $0 in the prior year. The increase in general and administrative expenses in the year ended December 31, 2011 was primarily due to the increase in business activities.
Liquidity and Capital Resources
Our independent registered public accountants have expressed the opinion that our financial condition raises substantial doubt about our ability to continue as a going concern. As of December 31, 2013, we had approximately $145,000 in cash. We maintain our cash in a checking and savings account on deposit with a large banking institution. We currently do not invest in any short term commercial paper or short-term certificates of deposit.
Research Loan
In 2013, we were awarded a research loan from the Alzheimer’s Drug Discovery Foundation. The award was funded in two installments of $102,630 totaling $205,260. We issued promissory notes evidencing each installment of the loan. The notes accrue interest at the rate of 3.25% per annum, and mature on January 21, 2017 and September 12, 2017, respectively. In connection with the award we also issued to the Alzheimer’s Drug Discovery Foundation a warrant to purchase 15,596 shares of the Company’s common stock at an exercise price of $0.99 per share. The terms of the award generally require us to apply the loan proceeds towards research on potential treatments for Alzheimer’s disease.
40
Cash Flows from Operating Activities
Net cash used in operating activities for the years ended December 31, 2013, 2012 and 2011 was $705,527, $1,387,245, and $285,218, respectively. Cash was used in operations for the year ended December 31, 2013 primarily due to our reported net loss of $872,641 and was offset by the increase in restricted cash of $79,065, a $52,732 increase in accrued liabilities due to the timing of invoices received after the year end and a $22,269 increase in accounts payable due to the timing of payments for vendor invoices. The cash used in operations in the year ended December 31, 2012 was primarily due to our reported net loss of $1,471,089 offset by the $32,376 increase in accounts payable due to the timing of payments for vendor invoices, the $29,910 stock based compensation add back and a $16,903 increase in accrued liabilities related to consulting expenses incurred but not invoiced as of the year end date. The cash used in operations in the year ended December 31, 2011 was primarily due to our reported net loss of $291,741.
Cash Flows from Investing Activities
Net cash used in investing activities for the years ended December 31, 2013, 2012 and 2011 was $206,448, $3,496, and $4,915, respectively. The cash used in investing activities in the year ended December 31, 2013 was primarily due to the restriction on cash relating to the grants from the Alzheimer’s Drug Discovery Foundation. Investing activities for the fiscal years ended December 31, 2012 and 2011 all related to cash paid for the purchases of property and equipment.
Cash Flows from Financing Activities
Net cash provided by financing activities in the years ended December 31, 2013, 2012 and 2011 was $179,051, $1,749,972, and $805,722, respectively. Cash provided by financing activities for the year ended December 31, 2013 consisted of $205,260 in proceeds from the grant received from the Alzheimer’s Drug Discovery Foundation, offset by the $26,209 in offering costs related to the issuance of Series B Preferred Stock in 2014. Cash provided by financing activities for the year ended December 31, 2012 consisted of $1,749,972 in proceeds received from the issuance of Series A Preferred Stock. Cash provided by financing activities for the year ended December 31, 2011 consisted of $805,722 of proceeds received from the issuance of Series A Preferred Stock.
Comparison of Three Months Ended September 30, 2014 and 2013
Operating Expenses
Research and development expenses were $159,883 for the three months ended September 30, 2014 compared to $103,210 in the prior year, a $56,673 increase, or 55%. The increase in research and development expenses in the three months ended September 30, 2014, was primarily due to a $52,730 increase in consultant’s costs due to an increase in the number of consultants retained and increased compensation levels in the current year period as compared to the prior year period.
General and administrative expenses were $246,182 in the three months ended September 30, 2014 compared to $64,321 in the prior year, a $181,861 increase. The increase in general and administrative expenses in the three months ended September 30, 2014, was primarily due to a $169,876 increase in compensation costs due increased headcount of two employees in the current year as compared to the prior year.
Comparison of Nine Months Ended September 30, 2014 and 2013
Operating Expenses
Research and development expenses were $405,215 for the nine months ended September 30, 2014 compared to $376,272 in the prior year, a $28,943 increase, or 8%. The increase in research and development
41
expenses in the nine months ended September 30, 2014, was primarily due to a $74,253 increase in consultant costs due to an increase in the number of consultants retained and increased compensation levels in the current year period as compared to the prior year period and a $65,874 increase in spending related to the grant from the Alzheimer’s Drug Discovery Foundation offset by lower salary and benefit costs of $63,756 due to a lower headcount of 2 employees in 2014 as compared to 2013 and a $48,548 decrease in lab services and supplies due to lower overall usage in 2014 as compared to 2013.
General and administrative expenses were $914,399 in the nine months ended September 30, 2014 compared to $259,639 in the prior year, a $654,760 increase. The increase in general and administrative expenses in the nine months ended September 30, 2014, was primarily due to a $498,096 increase in salary, benefit and stock based compensation costs due to an increase in headcount of 1 employee and the timing of stock option expense related to the granting of options and warrants in the current year period as compared to the prior year period, a $65,704 increase in outside services relating to recruiting costs as we source for new hires and a $39,530 increase in accounting fees relating to the costs of compliance.
Liquidity and Capital Resources
As of September 30, 2014, we had $1,818,843 in cash. We maintain our cash in a checking and savings account on deposit with a large banking institution. We currently do not invest in any short term commercial paper or short-term certificates of deposit.
Cash Flows from Operating Activities. Net cash used in operating activities for the nine months ended September 30, 2014 and 2013 was $719,393 and $560,965, respectively. Cash was used in operations for the nine months ended September 30, 2014 primarily due to our reported net loss of $1,324,599 offset by the increase in stock based compensation costs of $239,760, the $189,542 increase in accrued liabilities due to the timing of vendor invoices received and the $122,140 restricted cash relating to the use of the grant received from the Alzheimer’s Drug Discovery Foundation. The cash used in operations for the nine months ended September 30, 2013 was primarily due to our reported net loss of $637,881.
Cash Flows from Investing Activities. Net cash used in investing activities for the nine months ended September 30, 2014 and 2013 was $0 and $205,260, respectively. Investing activities for the nine months ended September 30, 2013 was due to the restrictions on cash relating to the grant from the Alzheimer’s Drug Discovery Foundation.
Cash Flows from Financing Activities. Net cash provided by financing activities in the nine months ended September 30, 2014 and 2013 was $2,393,066 and $204,475, respectively. Cash provided by financing activities for the nine months ended September 30, 2014 consisted of $2,640,079 in net proceeds from the issuance of Series B Preferred Stock offset by $247,013 in deferred offering costs relating to the Company’s initial public offering. Cash provided by financing activities for the nine months ended September 30, 2013, was due to the grant received from the Alzheimer’s Drug Discovery Foundation.
We expect to receive $2,700,000 in gross proceeds from the issuance of units pursuant to the exercise of our Put Rights. Together with the net proceeds of this offering we believe that we have sufficient cash to meet our working capital needs and operating expenses, including having at least $100,000 in unallocated funds, for at least the next 12 months. However, if unanticipated difficulties arise we may be required to raise additional capital to support our operations or curtail our research and development activities until such time as additional capital becomes available.
Off-Balance Sheet Arrangements.
We do not have any off-balance sheet arrangements.
42
Contractual Obligations
Licensing Agreements
Effective November 30, 2011, the Company entered into an Exclusive License Agreement (the “2011 Exclusive Agreement”) with the Regents of the University of California (the “Regents”) whereby the Regents granted to the Company an exclusive license for the use of certain patents. The Company paid the Regents an initial license issue fee of $35,000, which was charged to General and Administrative expense, as incurred. The Company agreed to pay the licensors specified development milestone payments aggregating up to $765,000 for the first product sold under the license. Milestone payments for additional products developed and sold under the license are reduced by 50%. The Company is also required to pay annual maintenance fees to the licensors. Aggregate maintenance fees for the first five years following execution of the agreement are $80,000. Thereafter, the Company is required to pay maintenance fees of $50,000 annually until the first sale of a licensed product. In addition, for the duration of the 2011 Exclusive Agreement, the Company is required to pay the licensors royalties equal to 2% of its worldwide net sales of drugs, therapies or other products developed from claims covered by the licensed patents, subject to a minimum royalty payment of $75,000 annually, beginning after the first commercial sale of a licensed product. The Company is required to pay royalties ranging from 8% of worldwide sublicense sales of covered products (if the sublicense is entered after commencement of phase II clinical trials) to 12% of worldwide sublicense sales (if the sublicense is entered prior to commencement of phase I clinical trials). The agreement also requires the Company to meet certain diligence and development milestones, including filing of an Investigational New Drug (“IND”) Application for a product covered by the agreement on or before the seventh anniversary of the agreement date. Through September 30, 2014, no royalties have been incurred under the 2011 Exclusive Agreement.
Effective August 6, 2013, the Company entered into an Exclusive License Agreement (the “2013 Exclusive Agreement”) with the Regents whereby the Regents granted to the Company an exclusive license for the use of certain other patents. The Company paid Regents an initial license issue fee of $10,000 for these other patents, which was charged to General and Administrative expense, as incurred. The Company agreed to pay the Regents specified development milestone payments aggregating up to $765,000 for the first product sold under the 2013 Exclusive Agreement. Milestone payments for additional products developed and sold under the 2013 Exclusive Agreement are reduced by 50%. In addition, for the duration of the 2013 Exclusive Agreement, the Company is required to pay the Regents royalties equal to 2% of the Company’s worldwide net sales of drugs, therapies or other products developed from claims covered by the licensed patent, subject to a minimum royalty payment of $75,000 annually, beginning after the first commercial sale of a licensed product. The Company is required to pay the Regents royalties ranging from 8% of worldwide sublicense sales of covered products (if the sublicense is entered after commencement of phase II clinical trials to 12% of worldwide sublicense sales (if the sublicense is entered prior to commencement of phase I clinical trials). The agreement also requires the Company to meet certain diligence and development milestones, including filing of an IND Application for a product covered by the agreement on or before the seventh anniversary of the agreement date. Through September 30, 2014, no royalties have been incurred under the 2013 Exclusive License Agreement.
Operating Lease
The Company rents laboratory space on a month to month basis in Pasadena, California. Rent expense amounted to $25,200, $24,600 and $6,800 for the years ended December 31, 2013, 2012 and 2011, respectively. Rent expense amounted to $5,400 in each of the three month periods ended September 30, 2014 and 2013. Rent expense amounted to $16,200 and $19,200 for the nine month periods ended September 30, 2014 and 2013, respectively.
Research Loan
In 2013, we were awarded a research loan from the Alzheimer’s Drug Discovery Foundation in the aggregate original principal amount of $205,260. The principal amount, together with interest accrued through maturity of approximately $28,000, is payable at maturity in 2017.
43
Recent Accounting Pronouncements
Under the JOBS Act, emerging growth companies are permitted to delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
In June 2014, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2014-10, “Development Stage Entities (Topic 915): Elimination of Certain Financial Reporting Requirements, Including an Amendment to Variable Interest Entities Guidance in Topic 810, Consolidation.” This ASU removes the definition of a development stage entity from the ASC, thereby removing the financial reporting distinction between development stage entities and other reporting entities from GAAP. In addition, the ASU eliminates the requirements for development stage entities to (1) present inception-to-date information in the statements of operations, cash flows, and shareholders’ deficit, (2) label the financial statements as those of a development stage entity, (3) disclose a description of the development stage activities in which the entity is engaged, and (4) disclose in the first year in which the entity is no longer a development stage entity that in prior years it had been in the development stage. ASU 2014-10 is effective for annual periods beginning after December 15, 2014. ASU 2014-10 does allow early adoption for entities that have not yet issued financial statements. The Company has early adopted ASU 2014-10 and reflected this adoption in its financial statement presentation contained herein.
The FASB has issued ASU No. 2014-12, Compensation – Stock Compensation (Topic 718): Accounting for Share-Based Payments When the Terms of an Award Provide That a Performance Target Could Be Achieved after the Requisite Service Period. This ASU requires that a performance target that affects vesting, and that could be achieved after the requisite service period, be treated as a performance condition. As such, the performance target should not be reflected in estimating the grant date fair value of the award. This update further clarifies that compensation cost should be recognized in the period in which it becomes probable that the performance target will be achieved and should represent the compensation cost attributable to the period(s) for which the requisite service has already been rendered. The amendments in this ASU are effective for annual periods and interim periods within those annual periods beginning after December 15, 2015. Earlier adoption is permitted. The adoption of this standard is not expected to have a material impact on the Company’s financial position and results of operations.
In August 2014, the FASB issued a new accounting standard which requires management to evaluate whether there is substantial doubt about an entity’s ability to continue as a going concern for each annual and interim reporting period. If substantial doubt exists, additional disclosure is required. This new standard will be effective for the Company for annual and interim periods beginning after December 15, 2016. Early adoption is permitted. The adoption of this pronouncement is not expected to have a material impact on the Company’s condensed financial statements.
Other recent accounting standards that have been issued or proposed by the FASB or other standards-setting bodies that do not require adoption until a future a date are not expected to have a material impact on the Company’s financial statements upon adoption.
Critical Accounting Policies
Our management’s discussion and analysis of our financial condition and results of operations are based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States (GAAP). GAAP requires us to make certain estimates and judgments that can affect the reported amounts of assets and liabilities as of the dates of the financial statements, the disclosure of contingencies as of the dates of the financial statements, and the reported amounts of revenue and expenses
44
during the periods presented. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities. If actual results or events differ materially from those contemplated by us in making these estimates, our reported financial condition and results of operations for future periods could be materially affected. See “Risk Factors” for certain matters that may affect our future financial condition or results of operations. An accounting policy is deemed to be critical if it requires an accounting estimate to be made based on assumptions about matters that are uncertain at the time the estimate is made, if different estimates reasonably could have been used, or if the changes in estimate that are reasonably likely to occur could materially impact the financial statements. Our management has discussed the development, selection and disclosure of these estimates with the audit committee of our board of directors.
The following critical accounting policies reflect significant judgments and estimates used in the preparation of our financial statements:
| • | Fair Value of Financial Instruments |
| • | Income Taxes |
| • | Share-based payment |
Fair Value of Financial Instruments
We measure the fair value of financial assets and liabilities based on the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. We utilize three levels of inputs that may be used to measure fair value:
Level 1 – quoted prices in active markets for identical assets or liabilities
Level 2 – quoted prices for similar assets and liabilities in active markets or inputs that are observable
Level 3 – inputs that are unobservable (for example, cash flow modeling inputs based on assumptions)
The carrying amounts of cash, accounts payable, accrued liabilities and debt approximate fair value due to the short-term nature of these instruments.
Income Taxes
We recognize deferred tax assets and liabilities for the expected future tax consequences of items that have been included or excluded in the financial statements or tax returns. Deferred tax assets and liabilities are determined on the basis of the difference between the tax basis of assets and liabilities and their respective financial reporting amounts (“temporary differences”) at enacted tax rates in effect for the years in which the temporary differences are expected to reverse. We assess the likelihood that deferred tax assets will be realized. To the extent that realization is not more likely than not, a valuation allowance is established. Based upon the Company’s losses since inception, management believes that it is more-likely-than-not that future benefits of deferred tax assets will not be realized and have recorded a full valuation allowance against our deferred tax assets.
Share-based Payment
We account for share-based payments using the fair value method. For employees and directors, the fair value of the award is measured on the grant date. For non-employees, fair value is generally measured based on the fair value of the services provided or the fair value of the common stock on the measurement date, whichever is more readily determinable and re-measured on interim financial reporting dates until the service is complete. We have historically granted stock options at exercise prices no less than the fair market value as determined by the board of directors, with input from management.
45
The weighted-average fair value of options and warrants has been estimated on the date of grant using the Black-Scholes pricing model. In computing the impact, the fair value of each instrument is estimated on the date of grant utilizing certain assumptions for a risk free interest rate, volatility and expected remaining lives of the awards. Since our shares have not been publicly traded, the fair value of stock-based payment awards was estimated using a volatility derived from an index of comparable entities. The assumptions used in calculating the fair value of share-based payment awards represent management’s best estimates, but these estimates involve inherent uncertainties and the application of management judgment. As a result, if factors change and we use different assumptions, our stock-based compensation expense could be materially different in the future. In addition, we are required to estimate the expected forfeiture rate and only recognize expense for those shares expected to vest. In estimating our forfeiture rate, we analyzed our historical forfeiture rate, the remaining lives of unvested options, and the number of vested options as a percentage of total options outstanding. If our actual forfeiture rate is materially different from our estimate, or if we reevaluate the forfeiture rate in the future, the stock-based compensation expense could be significantly different from what we have recorded in the current period. See Note 3 “Summary of Significant Account Policies – Share-Based Payment” to our Financial Statements for the years ended December 31, 2013, 2012 and 2011 included in the registration statement of which this prospectus forms a part for further information regarding the specific assumptions used with respect to stock-based compensation for the periods presented.
Since January 1, 2011, we granted stock options with exercise prices as follows:
| Grant Date |
Number of Shares Underlying Options |
Exercise Price Per Share |
Common Stock Fair Value Per Share on Date of Grant |
|||||||||
| April 2, 2012 |
1,471,699 | $ | 0.05 | $ | 0.05 | |||||||
| April 9, 2014 |
1,061,248 | $ | 0.26 | $ | 0.18 | |||||||
| November 20, 2014 |
1,475,687 | $ | 0.73 | $ | 0.51 | |||||||
The fair value of the common stock underlying our stock options was determined by our board of directors, with all options granted to be exercisable at a price per share not less than the per share fair value of our common stock underlying those options on the date of grant. Our board of directors determined the fair value of our common stock on the date of grant based on a number of factors including:
| • | contemporaneous independent valuations; |
| • | our performance, growth rate and financial condition at the time of the option grant; |
| • | scientific progress; |
| • | amounts recently paid by investors for our preferred stock; |
| • | the market performance of comparable publicly traded companies; |
| • | the likelihood of achieving a liquidity event for the shares of common stock underlying these stock options; and |
| • | the rights, preferences and privileges of our preferred stock relative to those of our common stock. |
Discussion of specific valuation inputs for option grants
Options Granted April 2, 2012
Our board of directors conducted a valuation of our common stock on April 2, 2012 in connection with the stock options granted on that date. In its evaluation the board of directors considered the status of our research programs, the enterprise value applied in a recent preferred stock financing, the value of our assets (comprised of intellectual property rights and cash on hand), our capital structure (including the rights and preferences of the Series A preferred stock which was then outstanding), the present value of our future cash flows as an early research stage company, and the trading prices of comparable publicly traded companies.
46
The board of directors also considered additional factors, including our existing licensing agreement, intellectual property position, prospects for additional funding, the funding prospects and valuations of similar companies, the ability of the management team and uncertainties regarding the Company’s access to additional financing.
Based on all of these factors, the board of directors determined a fair value of our common stock to be $0.05 per share as of the April 2, 2012 grant date.
Options Granted April 9, 2014
Our board of directors also determined the valuation of our common stock in connection with options granted on April 9, 2014. In its evaluation the board of directors considered developments since the prior valuation date, including the completion of additional scientific research, the development of our intellectual property portfolio, the conversion of the previously outstanding Series A preferred stock into common stock, and the expected completion of a planned Series B preferred stock financing.
In determining the value of our common stock we applied a combination of two generally accepted approaches to determine enterprise value – the BackSolve method and the probability weighted expected return method. A risk-adjusted discount for lack of marketability is then applied to values under each approach and the values are weighted to arrive at a concluded equity value. The BackSolve method calculates the equity value based on prices applicable to recent transactions in the subject company’s shares. The Black-Scholes model is used to determine the implied equity value and to allocate the same value among various share classes based on their rights and preferences. The analysis assumed that the Company would raise between $3 and $4 million from the sale of its Series B preferred stock at a price of $0.50 per share. The probability weighted expected return method entails a forward-looking analysis of possible future outcomes available to the company, the estimation of a future and present values under each outcome, and application of a probability factor to each outcome as of the valuation date. The potential future outcomes typically considered are in the form of exit events such as a sale or merger, initial public offering, dissolution, or continued as a private entity. Key assumptions in this analysis included significant uncertainties related to our timeline to completion of an initial public offering, with the probability of completing an initial public offering during 2015 weighted between 10% and 30%. Discounts for lack of marketability of 35% and 30%, respectively, were applied to the BackSolve and probability weighted expected return method analyses. The resulting valuation was determined as the weighted average of the results of these approaches, with a 60% weighting assigned to the BackSolve analysis and a 40% weighting assigned to the probability weighted expected return analysis.
Based on all these factors, the board of directors determined a fair value of our common stock to be $0.26 per share.
Options Granted November 20, 2014
Our board of directors also determined the valuation of our common stock in connection with options granted on November 20, 2014. In its evaluation the board of directors considered developments since the prior valuation date, including the hiring of a Chief Scientific Officer, progress of our scientific research and progress towards completion of our initial public offering.
In determining the value of our common stock we applied a generally accepted approach to determine enterprise value – the Probability-weighted Expected Return Method (PWERM). A risk-adjusted discount for lack of marketability is applied to the value under the PWERM approach and the value is weighted to arrive at a concluded equity value. The PWERM method entails a forward-looking analysis of possible future outcomes available to the company, the estimation of future and present values under each outcome, and application of a probability factor to each outcome as of the valuation date. The valuation focused on an initial public offering of our units as the probable future outcome and considered the proposed pricing for the units in our initial public
47
offering and the implied value of the warrants included as part of the units. Given the progress toward completion of our initial public offering at $1.00 per unit, for the purposes of the PWERM analysis, we applied an 80% probability of completing our initial public offering during December, 2014, and a 20% probability of completing our initial public offering in January, 2015, and applied a discount for lack of marketability of approximately 19%.
Based on all these factors, the board of directors determined a fair value of our common stock to be $0.73 per share.
We believe the difference between the fair values of $0.26 and $0.73 per share of our common stock, as determined by the Board of Directors in April 2014 and November 2014, respectively, and the initial public offering price of $1.00 per unit now anticipated by our board of directors is a result of the following factors:
| • | The price per unit necessarily assumes that the initial public offering has occurred and a public market for our common stock has been created, and therefore excludes any marketability or illiquidity discount for our common stock; |
| • | Our board considered the fair value of one share of our common stock on each of the valuation dates. Each unit consists of one share of common stock and one half of one common stock purchase warrant. The anticipated price per unit necessarily attributes some value to the warrants included in the units; and |
| • | Significant uncertainties surrounding the initial public offering and difficulties anticipated in completing an initial public offering at our early stage of development. |
48
Overview
We are a research stage biotechnology company committed to applying our scientific leadership in the biology of Mitochondrial-Derived Peptides, or MDPs, to extend the healthy lifespan and transform the lives of patients with major diseases.
MDPs, which are peptides encoded within the mitochondrial genome, comprise a novel collection of bonded amino acids identified primarily by our founders and their research colleagues. To date, we have conducted investigational research into MDPs to evaluate their therapeutic potential through in vitro and in vivo models. Based on our research, we have identified four MDPs for possible advancement into drug candidate programs targeting one or more indications from a variety of diseases including cancer, Alzheimer’s disease, atherosclerosis and certain metabolic disorders such as Type 2 Diabetes and obesity.
We believe that the success of one of these possible MDP candidate programs, and further future development into a clinically effective therapeutic drug, while uncertain, could potentially address significant unmet medical needs. Given the age-related risk factors associated with these disease indications, an effective therapeutic drug could offer substantial improvements in the quality of life, longevity, and medical and care cost burden of our aging population.
We are the exclusive licensee from the Regents of the University of California and the Albert Einstein College of Medicine to four issued U.S. patents and four U.S. and international patent applications. Our licensed patents and patent applications are directed to compositions comprising MDPs and MDP analogs and methods of their use in the treatment of indicated diseases. See “Business – Patents and Other Intellectual Property”.
Our company was formed in 2007 and was converted to become a Delaware corporation in 2009.
Our Scientific Foundations
Our scientific leadership is centered on the expertise of our founders, Dr. Pinchas Cohen, Dean of the Davis School of Gerontology at the University of Southern California, and Dr. Nir Barzilai, Professor of Genetics and Director of the Institute for Aging Research at the Albert Einstein College of Medicine, and is supported by our co-founders, Dr. David Sinclair, Professor of Genetics at Harvard Medical School, and Dr. John Amatruda, former Senior Vice President and Franchise Head for Diabetes and Obesity at Merck Research Laboratories. Our founders and co-founders are widely considered to be scientific experts and thought leaders at the intersection of cellular and mitochondrial genetics and biology, the biology of aging, metabolism, and drug discovery, development and commercialization.
The scientific research in the areas of mitochondrial genomics and biology, age-related diseases, longevity, metabolism and MDPs underlying our founder’s discoveries and our intellectual property portfolio was conducted by Dr. Cohen, Dr. Barzilai and their academic collaborators with the support of research grants aggregating over $30 million awarded to their respective academic institutions since 2001 by the National Institutes of Health, private foundations, and other grant funding organizations. Our founders’ multi-disciplinary expertise and their investigations into and knowledge of age-related diseases has enabled and focused our Company’s research efforts on the mitochondrial genome and its potential to yield peptides for therapeutic advancement.
Our Opportunity
Mitochondria are components within the cell that produce energy and regulate cell death in response to signals received from the cell. They are the only cell components, other than the nucleus, that have their own genome. Until recently, scientists believed the mitochondrial genome was simple, containing only 37 genes. Research by our founders and their academic collaborators has revealed that the mitochondrial genome has as
49
many as 80 distinct new genes that encode peptides, only several of which have been characterized to date. We refer to these as Mitochondrial-Derived Peptides, or MDPs. MDPs influence cellular activities by acting as messengers between cells, triggering intra-cellular changes that affect cell growth and differentiation and play a role in metabolism.
MDPs represent a diverse and largely unexplored collection of peptides, which we believe have the potential to lead to novel therapeutics for a number of diseases with significant unmet medical needs. Although their mechanisms of action are not yet fully known, animal models have shown that MDPs have metabolic effects, neuroprotective effects, cytoprotective effects (protection against toxicity leading to cell death), and anti-inflammatory effects. For example, Humanin, the first MDP to be discovered (in 2001), has been shown to have protective effects in various disease models, including Alzheimer’s disease, atherosclerosis, myocardial and cerebral ischemia and Type 2 Diabetes. Humanin levels in humans have been shown to decline with age, while elevated levels of humanin are found in centenarians (those who live over 100 years), as well as their offspring.
Compared to the nuclear genome, which has been a subject of drug discovery efforts for decades, the mitochondrial genome has remained relatively unexplored. We believe that Cohbar is a first mover in exploring the mitochondrial genome to identify MDPs with potential to be developed into transformative medicines, and that the depth of our scientific expertise, together with our intellectual property portfolio, will enable us to sustain this competitive advantage. By augmenting our scientific leadership and MDP discoveries with drug discovery and development expertise and capabilities, we believe we can identify and develop MDP-based therapeutic candidates that harness MDP cell-signaling mechanisms and unlock the therapeutic potential of this collection of peptides.
Our Strategy
We aim to build a multi-product company based on our expertise in MDP biology that, independently or together with strategic partners, discovers, develops and commercializes first- and best-in-class medicines to treat a wide variety of diseases with large unmet medical need. Key elements of our strategy include:
| • | Exploiting our MDP discoveries to date by advancing research and development within our lead programs; |
| • | Continuing to leverage our expertise in MDP discovery to expand our pipeline of research peptides; |
| • | Expanding our intellectual property portfolio relevant to MDP-based therapeutics; |
| • | Supplementing and supporting our founders’ expertise and efforts with additional scientific leadership, staff and facilities; |
| • | Maintaining our competitive, first-mover advantage in the field of MDP-based therapeutics; |
| • | Leveraging relationships with academic partners and contract research organizations (CROs) to advance our research programs; and |
| • | Developing strategic partnerships with larger pharmaceutical companies and other organizations to support our research programs and future development and commercialization efforts. |
Our Lead Peptides
Our research efforts to date have focused on discovering and evaluating our MDPs for potential development as drug candidates. We seek to identify and advance research on MDPs with superior potential for yielding a drug candidate, and ultimately a drug, for which we have a strong intellectual property position. We also seek to take advantage of efficiencies that may be gained should a single MDP drug candidate prove effective for multiple indications. Based on our evaluation of MDPs currently in our research pipeline we are actively engaged in research of four MDPs for potential advancement into drug candidate programs.
50
MOTS-c
MOTS-c is an MDP discovered in 2012 by our founders and their academic collaborators. To date, our laboratory and rodent studies indicate that MOTS-c plays a significant role in regulation of metabolism and we believe a MOTS-c analog has therapeutic potential for Type 2 Diabetes mellitus, as well as other diseases, such as obesity, fatty liver and certain cancers. We intend to advance research on MOTS-c and its analogs as our lead program because, based on our research to date, we believe that MOTS-c has the greatest potential, among our current MDPs, for development as a commercially successful drug. We also believe we have greater ability to develop a comprehensive portfolio of intellectual property around MOTS-c compared to our other research peptides.
MOTS-c acts in metabolic regulation by acting in signal processes that induce the cell to control its energy through uptake of glucose, oxidation of fatty acids or other cellular processes. MOTS-c appears to increase glucose usage and increase fatty acid oxidation, with the most notable effect in muscle, the major tissue responsible for glucose uptake.
Age dependent insulin resistance was reversed in aged mice treated with MOTS-c.
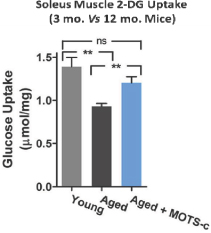
MOTS-c treatment of several lines of mice for four weeks curbed weight gain in non-diabetic mice fed a high-fat diet, without changing food intake, and reduced blood sugar levels in these animals.
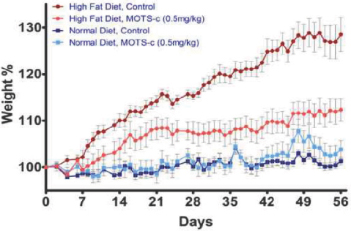
51
We consider Type 2 Diabetes to be a potential primary indication for a suitable MOTS-c analog because the above data and preliminary results of our other investigative research suggest that MOTS-c may provide a novel mechanism of action for effective glycemic control within diabetic populations, and that MOTS-c may avoid or even improve conditions associated with several current treatments for Type 2 Diabetes, such as weight gain. A MOTS-c analog may also prove useful in treatment of Type 2 Diabetes when combined with an existing or future therapy. We may, however, determine to advance a drug candidate based on a MOTS-c analog against a different or additional primary indications if we obtain data suggesting that a MOTS-c analog may have greater therapeutic potential against another indication (for example obesity, fatty liver, cancer or cardiovascular disease), or that the development, approval or commercialization pathway may be more favorable for a drug candidate targeted against an alternative indication.
Our research plan for MOTS-c includes additional in vivo animal studies, and the design and screening of additional analogs with peptide modifications designed to improve the stability, half-life and/or function of the candidate peptide.
Humanin
Humanin, the first MDP to be discovered, was discovered independently by three separate groups in 2001. Our founder Dr. Cohen headed one such group and, in 2003, published its discovery along with follow-up experiments demonstrating that humanin shows benefits in a rat model of Type 2 Diabetes.
The potential relevance of humanin in longevity and the prevalence of aging related diseases has been observed in studies of centenarians (people living to 100 years old) and their offspring. Centenarians have less or similar prevalence of certain diseases compared to people who are 20 to 30 years younger, and their offspring have less prevalence of certain diseases compared to their age group.
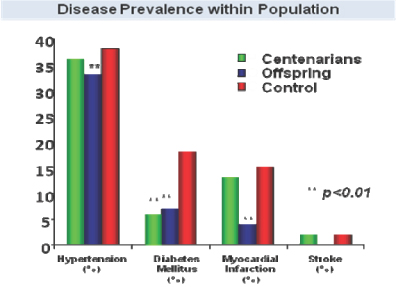
52
The development of assays to measure humanin levels in plasma enabled the observation of humanin levels in aged patients and patients with poor endothelial function associated with cardiovascular diseases. Humanin declines with age but is higher in the offspring of centenarians when compared to an age and gender matched control group. Humanin is lower in humans with poor endothelial function, a major risk factor for cardiovascular diseases.
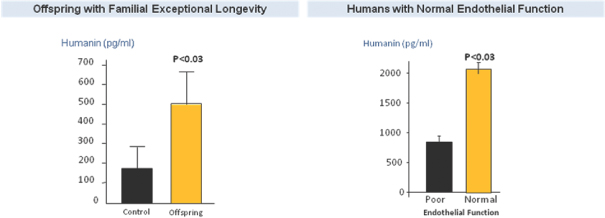
SHLP-6 and SHLP-2
We and our academic collaborators have discovered several other MDPs with properties related to humanin, which we refer to as small humanin-like peptides, or SHLPs. Of these MDPs, our investigational research of SHLP-6 and its potential for the treatment of cancer is the most advanced. SHLP-6 cancer treatment models conducted both in vitro in cancer cell lines and in vivo in tumor models of human prostate cancer in xenograft transplanted mice demonstrated suppression of cancer progression via a dual mechanism involving suppression of tumor angiogenesis (blood vessel development) as well as induction of apoptosis (cancer cell death), as shown below:
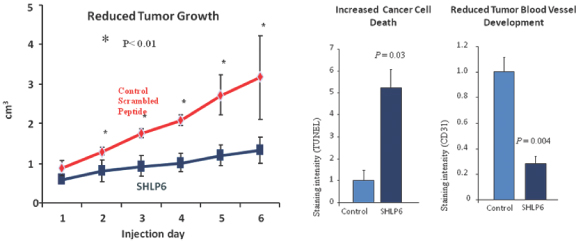
We consider SHLP-6 as our primary research peptide for the treatment of cancer and plan to advance our research on SHLP-6, or a suitable analog, in parallel with our MOTS-c research program.
53
We also have evidence that another of our MDPs, SHLP-2, as well as certain of our humanin analogs, may be useful in the treatment of Alzheimer’s disease. In vitro experiments have shown SHLP-2 and these humanin analogs to have protective effects against neuronal toxicity, and have demonstrated that SHLP-2 and the humanin analogs are transported through the blood-brain barrier. We consider SHLP-2, humanin and humanin analogs of potential interest for the development of MDP-based treatments for Alzheimer’s disease.
Our Target Indications
Our drug discovery efforts are centered on identification of Mitochondrial Derived Peptides that have therapeutic potential to be advanced as drug candidates. Our research programs to date suggest multiple possible therapeutic indications for each of our lead peptides. While we believe any drug candidates we identify would be advanced against one of the following diseases as a primary indication, it is possible that we may determine to advance a drug candidate for treatment of a different disease as a primary indication. We may determine to advance any future drug candidate against an alternative primary disease indication if, for example, additional data suggests greater therapeutic potential for the drug candidate against the alternative indication, or we determine that the development, approval or commercialization pathway may be more favorable for a drug candidate targeted against the alternative indication.
Type 2 Diabetes – Type 2 Diabetes is a chronic disease characterized by a relative deficiency in insulin production and secretion by the pancreas and an inability of the body to respond to insulin normally, i.e. insulin resistance. Hyperglycemia, or raised blood sugar, is a common effect of uncontrolled diabetes and over time leads to serious damage to many of the body’s systems, especially the nerves, kidneys, eyes and blood vessels. The World Health Organization reports that over 346 million people worldwide suffer from diabetes, of which 90% is Type 2 Diabetes.
Cancer – Cancer is a generic term for a large group of diseases that can affect any part of the body. One defining feature of cancer is the rapid creation of abnormal cells that grow beyond their usual boundaries, and which can then invade adjoining parts of the body and spread to other organs. This process is referred to as metastasis. Metastases are a major cause of death from cancer. Cancer is a leading cause of death worldwide. The World Health Organization estimates that in 2012 there were 14.1 million new cancer cases diagnosed, 8.2 million cancer deaths and 32.6 million people living with cancer (within 5 years of diagnosis) worldwide. Cancer drugs such as chemotherapy, hormone therapy and other treatments are used to destroy cancer cells. The goal of cancer drugs is to cure the disease or, when a cure is not possible, to prolong life or improve quality of life for patients with incurable cancer. According to the IMS institute for Healthcare Informatics, global spending on cancer treatment was approximately $91 billion in 2013.
Alzheimer’s disease – In the brain, neurons connect and communicate at synapses, where tiny bursts of chemicals called neurotransmitters carry information from one cell to another. Alzheimer’s disrupts this process and eventually destroys synapses and kills neurons, damaging the brain’s communication network. The Alzheimer’s Association® reports that an estimated 5.2 million Americans suffered from Alzheimer’s disease in 2013, and that by 2025 an estimated 7.1 million Americans will be afflicted by the disease, a 40 percent increase from currently affected patients. There is no cure, and medications on the market today treat only the symptoms of Alzheimer’s disease and do not have the ability to stop its onset or its progression. There is an urgent and unmet need for both a disease-modifying drug for Alzheimer’s disease as well as for better symptomatic treatments.
Atherosclerosis – Atherosclerosis is commonly referred to as a “hardening” or furring of the arteries. It is caused by the formation of multiple atheromatous plaques within the arteries. This process is the major underlying risk for developing myocardial infarction (heart attack) as those plaques will either narrow the vessel or rupture, preventing blood flow in the coronary artery to parts of the heart muscle. Heart disease is the leading cause of death for both men and women. Coronary heart disease is the most common type of heart disease,
54
killing nearly 380,000 people annually. Cholesterol lowering drugs are considered the main preventive approach to treat atherosclerosis, however these drugs are estimated to prevent only one-third of incidences of myocardial infarction, and there is significant unmet need for additional therapeutic options.
Manufacturing and Supply
We expect to engage one or more third-party contract manufacturers, or CMOs, to produce a clinical supply of peptides in accordance with current good manufacturing practices (cGMPs) at higher purity levels. Peptide-based drugs are produced using a chemical synthesis process, and historically have been simpler and less expensive to manufacture than biologic drugs from cell-based sources. CMOs will be selected based on results of demonstration syntheses, regulatory track record, commercial manufacturing and control experience, staff experience, training and skill, intellectual property considerations and price.
Competition
The biotechnology and pharmaceutical industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. While we believe that our scientific knowledge, technology, and development experience provide us with competitive advantages, we face potential competition from many different sources, including major pharmaceutical, specialty pharmaceutical and biotechnology companies, academic institutions and governmental agencies and public and private research institutions. Any product candidates that we successfully develop and commercialize will compete with existing therapies and new therapies that may become available in the future.
Many of our competitors may have significantly greater financial resources and capabilities for research and development, manufacturing, pre-clinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Mergers and acquisitions in the pharmaceutical, biotechnology and diagnostic industries may result in even more resources being concentrated among a smaller number of our competitors. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs. Small or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies.
The key competitive factors affecting the success of all of our product candidates, if approved, are likely to be their efficacy, safety, convenience, price and the availability of reimbursement from government and other third-party payors.
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any products that we may develop. Our competitors also may obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market. In addition, our ability to compete may be affected in many cases by insurers or other third-party payors seeking to encourage the use of products which are generic or are otherwise less expensive to provide.
There are numerous therapies currently marketed to treat diabetes, cancer and Alzheimer’s disease. These therapies are varied in their design, therapeutic application and mechanism of action and may provide significant competition for any of our product candidates for which we obtain market approval. New products may also become available that provide efficacy, safety, convenience and other benefits that are not provided by currently marketed therapies. As a result, they may provide significant competition for any of our product candidates for which we obtain market approval.
55
If MOTS-c or analogs of MOTS-c are developed and approved for treatment of patients with diabetes, it would compete with several classes of drugs for Type 2 Diabetes that are approved to improve glucose control, including sulfonylureas, PPAR gamma agonists, biguanides, alpha glucosidase inhibitors, DPP IV inhibitors, GLP1 agonists, SGLT2 inhibitors, bromocriptine and insulin. Insulin sensitizing agents approved to treat Type 2 Diabetes are the PPAR gamma agonists pioglitazone and rosiglitazone. These agents are not generic, are oral once daily pills and are effective in lowering glucose and A1C. Metformin is also sometimes called an insulin sensitizer. It is available as a generic and comes in a once daily formulation. Drugs approved for obesity may also be used to treat Type 2 Diabetes. In addition there are several investigational drugs being studied to treat Type 2 Diabetes and if these investigational therapies were approved they would also compete with MOTS-c.
If SHLP-6 (or MOTS-c) is developed and approved for treatment of patients with cancer, it would compete with all approved therapies for the cancer it is approved to treat. Since the specific cancer that these investigational therapies might be approved to treat is unknown, they would theoretically compete with any pharmaceutical agent that is approved to treat cancer. In addition there are several investigational drugs being studied to treat cancer and if these investigational therapies were approved they would also compete with SHLP-6 and MOTS-c.
If SHLP-2 (or humanin) is developed and approved for treatment of patients with Alzheimer’s disease, it would compete with all approved therapies to treat Alzheimer’s disease including donepezil (Aricept), galantamine (Razadyne), memantine (Namenda), rivastigmine (Exelon) and tacrine (Cognex). In addition, there are several investigational drugs being studied to treat Alzheimer’s that, if approved, would also compete with SHLP-2 or humanin.
Research and Development
Our research activities related to development of MDPs include investigational research to evaluate the therapeutic potential of certain discovered MDPs, in vitro experiments, and analog engineering of certain discovered MDPs to improve their characteristics as potential drug development candidates, and in vivo animal studies. Depending on factors of capability, cost, efficiency and intellectual property rights our research activities are conducted independently at our laboratory facility, by CROs pursuant to contractual arrangements, or under research arrangements with academic institutions.
Our research staff and laboratory facility are dedicated primarily to investigative research on our identified MDPs. Within this setting we are able to utilize the specialized expertise of our scientific staff and consultants to advance our research programs within the field of MDP biology and chemistry.
From time to time we enter into research arrangements with academic laboratories, particularly in circumstances where collaboration with an academic laboratory will enable us to more efficiently and effectively access equipment, expertise or other capabilities necessary to advance our research programs.
We also contract with CROs to conduct certain activities within our research programs, particularly in vivo experiments. We evaluate CROs based on their ability to provide consistently well staffed facilities and their experience in successfully completing similar experiments.
Our research and development expenses were $478,256, $854,292 and $109,301 in fiscal years 2013, 2012 and 2011, respectively, and $405,215 and $376,272 in the nine months ended September 30, 2014 and 2013, respectively.
Patents and other Intellectual Property
Our commercial success depends in part on our ability to obtain and maintain proprietary protection for our novel biological discoveries and therapeutic methods, to operate without infringing on the proprietary rights of others and to prevent others from infringing our proprietary rights. Our policy is to seek to protect our proprietary position by, among other methods, filing U.S. and foreign patent applications related to our proprietary
56
technology, inventions and improvements that are important to the development and implementation of our business. We also rely on trade secrets, know-how, continuing technological innovation and potential in-licensing opportunities to develop and maintain our proprietary position.
Our intellectual property and patent strategy is focused on our MDP-based therapeutics. We typically seek composition-of-matter and method-of-treatment patents for our MDPs based on pre-clinical evaluation of therapeutic potential. We believe that the opportunity to engineer analogs or create combination therapies will afford us the opportunity to strengthen IP protection for our drug development candidates as they advance through our development pipeline and to broaden our IP protection internationally.
Individual patents extend for varying periods of time depending on the date of filing of the patent application or the date of patent issuance and the legal term of patents in the countries in which they are obtained. Generally, patents issued from applications filed in the United States are effective for twenty years from the earliest non-provisional filing date. In addition, in certain instances, a patent term can be extended to recapture a portion of the term effectively lost as a result of the FDA regulatory review period, however, the restoration period cannot be longer than five years and the total patent term, including the restoration period, must not exceed 14 years following FDA approval. The duration of foreign patents varies in accordance with provisions of applicable local law, but typically is also twenty years from the earliest international filing date. We currently have exclusive rights to four issued patents that will expire starting in 2028.
A summary of our patent estate as it relates to our lead research peptides appears below:
| Therapeutic Activities / Method of Use Claims | ||||||||||||||||||
| Granted / Filed |
Composition Claims |
Type 1 Diabetes |
Type 2 Diabetes |
Obesity | Fatty Liver |
Cancer | Alzheimer’s | Atherosclerosis | ||||||||||
| MOTS-c | Filed | ü | ü | ü | ü | ü | ü | |||||||||||
| SHLP-6 | Filed | ü | ü | |||||||||||||||
| SHLP-2 | Granted | ü | ü | ü | ü | |||||||||||||
| Humanin Analogs |
Granted | ü | ü | |||||||||||||||
| Humanin Analogs |
Two Granted |
ü | ||||||||||||||||
| Humanin and Humanin Analogs |
Filed | ü | ||||||||||||||||
National and international patent laws concerning peptide therapeutics remain highly unsettled. Policies regarding the patent eligibility or breadth of claims allowed in such patents are currently in flux in the United States and other countries. Changes in either the patent laws or in interpretations of patent laws in the United States and other countries can diminish our ability to protect our inventions and enforce our intellectual property rights. Accordingly, we cannot predict the breadth or enforceability of claims that may be granted in our patents or in third-party patents. The biotechnology and pharmaceutical industries are characterized by extensive litigation regarding patents and other intellectual property rights. Our ability to maintain and solidify our proprietary position for our drugs and technology will depend on our success in obtaining effective claims and enforcing those claims once granted. We do not know whether any of the patent applications that we may file or license from third parties will result in the issuance of any patents. The issued patents that we license, or may license or own in the future, may be challenged, invalidated or circumvented, and the rights granted under any issued patents may not provide us with sufficient protection or competitive advantages against competitors with similar technology. Furthermore, our competitors may be able to independently develop and commercialize
57
similar drugs or duplicate our technology, business model or strategy without infringing our patents. Because of the extensive time required for clinical development and regulatory review of a drug we may develop, it is possible that, before any of our drugs can be commercialized, any related patent may expire or remain in force for only a short period following commercialization, thereby reducing any advantage of any such patent. The patent positions for our lead research peptides are described below:
MOTS-c Patent Coverage
We are the exclusive licensee from the Regents of the University of California (the “Regents”) to intellectual property rights related MOTS-c, including one patent application filed in the United States (U.S. Application No. 14/213,617) and one international patent application filed concurrently (PCT/US2014/28968). Both applications include claims directed to the MOTS-c composition of matter, as well as methods of using MOTS-c to treat Type 1 Diabetes, Type 2 Diabetes, fatty liver, obesity and cancer.
SHLP-2 and SHLP-6 Patent Coverage
We are the exclusive licensee from the Regents to intellectual property for SHLP-2 and SHLP-6. This intellectual property includes the following issued and pending patents:
| • | U.S. patent No. 8,637,470, issued on January 28, 2014, with claims directed to the SHLP-2 composition of matter, and variants with therapeutic activity for treating Alzheimer’s disease and Types 1 and 2 Diabetes. |
| • | A divisional patent application in the United States for SHLP-6 (U.S. Application No. 14/134,430), with claims directed at the SHLP-6 composition of matter, and methods of use in treating cancer. |
We have limited ability to expand coverage of this patent family outside of the United States, however we have developed and are currently evaluating analogs of SHLP-2 and SHLP-6 with modifications that improve potency or bioactivity of the peptide. If our evaluations show efficacy of these or other analog SHLPs in animal models, then we anticipate filing additional patents covering the composition of the modified SHLP and its methods of use in the United States, and internationally based on our evaluation of the potential for commercialization in those markets.
Humanin and Humanin Analogs Patent Coverage
We are the exclusive licensee from the Regents and the Albert Einstein College of Medicine of Yeshiva University to U.S. patent applications and issued U.S. patents and covering humanin and humanin analogs for treatment of disease.
| • | U.S. Patent No. 8,309,525, issued on November 13, 2012, with claims covering pharmaceutical compositions of humanin analogs for increasing insulin sensitivity. |
| • | U.S. Patent No. 7,998,928, issued on August 16, 2011, with claims directed to methods of using a humanin analog to treat Type 1 Diabetes. |
| • | U.S. Patent No. 8,653,027 issued on February 18, 2014 as a continuation of U.S. Patent 7,998,928, with claims directed to methods of using an additional humanin analog to treat Type 1 Diabetes. |
| • | U.S. Patent Application No. 13/526,309 (pending), with claims directed to methods of using humanin or a humanin analog to treat atherosclerosis. |
Trade Secrets
In addition to patents, we rely upon unpatented trade secrets and know-how and continuing technological innovation to develop and maintain our competitive position. We seek to protect our proprietary information, in part, using confidentiality agreements with our commercial partners, collaborators, employees and consultants and invention assignment agreements with our employees. These agreements are designed to protect our
58
proprietary information and, in the case of the invention assignment agreements, to grant us ownership of technologies that are developed through a relationship with a third party. These agreements may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our commercial partners, collaborators, employees and consultants use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions.
Trademark
We have applied for registration of the trademark COHBARTM in the United States.
In-licenses
MOTS-c Exclusive License
On August 6, 2013, we entered into an exclusive license agreement with the Regents to obtain worldwide, exclusive rights under patent filings and other intellectual property rights in inventions developed by Dr. Cohen and academic collaborators at the University of California, Los Angeles. The intellectual property includes the pending U.S. and international patent filings described above under “MOTS-c Patent Coverage”.
We agreed to pay the Regents specified development milestone payments aggregating up to $765,000 for the first product sold under the license. Milestone payments for additional products developed and sold under the license are reduced by 50%. In addition, we are required to pay the Regents royalties equal to 2% of our worldwide net sales of drugs, therapies or other products developed from claims covered by the licensed patent, subject to a minimum royalty payment of $75,000 annually, beginning after the first commercial sale of a licensed product. We are required to pay the Regents royalties ranging from 8% of worldwide sublicense sales of covered products (if the sublicense is entered after commencement of phase II clinical trials) to 12% of worldwide sublicense sales (if the sublicense is entered prior to commencement of phase I clinical trials). The agreement also requires us to meet certain diligence and development milestones, including filing of an IND for a product covered by the agreement on or before the seventh anniversary of the agreement date.
Under the agreement, the license rights granted to us are subject to any rights the United States Government may have in such licensed rights due to its sponsorship of research that led to the creation of the licensed rights. The agreement also provides that if the Regents become aware of a third-party’s interest in exploiting the licensed technologies in a field that we are not actively pursuing, then we may be obligated either to issue a sublicense for use in the unexploited field to the third-party on substantially similar terms or to actively pursue the unexploited field subject to appropriate diligence milestones. The agreement terminates upon the expiration of the last valid claim of the licensed patent rights. We may terminate the agreement at any time by giving the Regents advance written notice. The agreement may also be terminated by the Regents in the event of our continuing material breach after notice of such breach and the opportunity to cure.
Humanin and SHLPs Exclusive License
On November 30, 2011, we entered into an exclusive license agreement with the Regents and the Albert Einstein College of Medicine at Yeshiva University to obtain worldwide, exclusive rights under patent filings and other intellectual property rights in inventions developed by Drs. Cohen and Barzilai and their academic collaborators. The intellectual property subject to the agreement includes four issued and two pending U.S. patents including composition claims directed to humanin analogs, SHLP-2 and SHLP-6 and methods of use claims directed to humanin, humanin analogs and SHLP-6. See “Humanin and Humanin Analogs Patent Coverage” and “SHLP-2 and SHLP-6 Patent Coverage”.
We agreed to pay the licensors specified development milestone payments aggregating up to $775,000 for the first product sold under the license. Milestone payments for additional products developed and sold under the
59
license are reduced by 50%. We are also required to pay annual maintenance fees to the licensors. Aggregate maintenance fees for the first five years following execution of the agreement are $80,000. Thereafter, we are required to pay maintenance fees of $50,000 annually until the first sale of a licensed product. In addition, we are required to pay the licensors royalties equal to 2% of our worldwide net sales of drugs, therapies or other products developed from claims covered by the licensed patents, subject to a minimum royalty payment of $75,000 annually, beginning after the first commercial sale of a licensed product. We are required to pay royalties ranging from 8% of worldwide sublicense sales of covered products (if the sublicense is entered after commencement of phase II clinical trials) to 12% of worldwide sublicense sales (if the sublicense is entered prior to commencement of phase I clinical trials). The agreement also requires us to meet certain diligence and development milestones, including filing of an IND for a product covered by the agreement on or before the seventh anniversary of the agreement date.
Under the agreement, the license rights granted to us are subject to any rights the United States Government may have in such licensed rights due to its sponsorship of research that led to the creation of the licensed rights. The agreement terminates upon the expiration of the last valid claim of the licensed patent rights. We may terminate the agreement at any time by giving the Regents advance written notice. The agreement may also be terminated by the Regents in the event of our continuing material breach after notice of such breach and the opportunity to cure.
Government Regulation
The pre-clinical studies and clinical testing, manufacture, labeling, storage, record keeping, advertising, promotion, export, marketing and sales, among other things, of our therapeutic candidates and future products, are subject to extensive regulation by governmental authorities in the United States and other countries. In the United States, pharmaceutical products are regulated by the FDA under the Federal Food, Drug, and Cosmetic Act and other laws. Biologics are subject to regulation by the FDA under the FDCA, the Public Health Service Act, and related regulations, and other federal, state and local statutes and regulations. Biological products include, among other things, viruses, therapeutic serums, vaccines and most protein products. Product development and approval within these regulatory frameworks takes a number of years, and involves the expenditure of substantial resources.
Regulatory approval will be required in all major markets in which we, or our licensees, seek to test our products in development. At a minimum, such approval requires evaluation of data relating to quality, safety and efficacy of a product for its proposed use. The specific types of data required and the regulations relating to these data differ depending on the territory, the drug involved, the proposed indication and the stage of development.
In general, new chemical entities are tested in animals to determine whether the product is reasonably safe for initial human testing. Clinical trials for new products are typically conducted in three sequential phases that may overlap. Phase 1 trials typically involve the initial introduction of the pharmaceutical into healthy human volunteers and the emphasis is on testing for safety, dosage tolerance, metabolism, distribution, excretion and clinical pharmacology. In the case of serious or life-threatening diseases, such as cancer, initial Phase 1 trials are often conducted in patients directly, with preliminary exploration of potential efficacy. Phase 2 trials involve clinical trials to evaluate the effectiveness of the drug for a particular indication or indications in patients with the disease or condition under study and to determine the common short-term side effects and risks associated with the drug. Phase 2 trials are typically closely monitored and conducted in a relatively small number of patients, usually involving no more than several hundred subjects. Phase 3 trials are generally expanded, well-controlled clinical trials. They are performed after preliminary evidence suggesting effectiveness of the drug has been obtained, and are intended to gather the additional information about effectiveness and safety that is needed to evaluate the overall benefit-risk relationship of the drug and to provide an adequate basis for physician labeling.
In the U.S., specific pre-clinical data, chemical data and a proposed clinical study protocol, as described above, must be submitted to the FDA as part of an Investigational New Drug application, or IND, which, unless the FDA objects, will become effective 30 days following receipt by the FDA. Phase 1 trials may commence
60
only after the IND application becomes effective. Following completion of Phase 1 trials, further submissions to regulatory authorities are necessary in relation to Phase 2 and 3 trials to update the existing IND. Authorities may require additional data before allowing the trials to commence and could demand discontinuation of studies at any time if there are significant safety issues. In addition to regulatory review, a clinical trial involving human subjects has to be approved by an independent body. The exact composition and responsibilities of this body differ from country to country. In the U.S., for example, each clinical trial is conducted under the auspices of an Institutional Review Board at the institution at which the clinical trial is conducted. This board considers among other things, the design of the clinical trial, ethical factors, the safety of the human subjects and the possible liability risk for the institution.
Information generated in this process is susceptible to varying interpretations that could delay, limit, or prevent regulatory approval at any stage of the approval process. Failure to demonstrate adequately the quality, safety and efficacy of a therapeutic drug under development would delay or prevent regulatory approval of the product.
In order to gain marketing approval, we must submit a new drug application, or NDA, for review by the FDA. The NDA requires information on the quality of the chemistry, manufacturing and pharmaceutical aspects of the product and non-clinical and clinical data.
There can be no assurance that if clinical trials are completed that we or any future collaborative partners will submit an NDA or similar applications outside the U.S. for required authorizations to manufacture or market potential products, or that any such applications will be timely reviewed or approved. Approval of an NDA can take several months to several years or be denied, and the approval process can be affected by a number of factors. Additional studies or clinical trials may be requested during the review and may delay marketing approval and involve unbudgeted costs. Regulatory authorities may conduct inspections of relevant facilities and review manufacturing procedures, operating systems and personnel qualifications. In addition to obtaining approval for each product, in many cases each drug manufacturing facility must be approved. Further, inspections may occur over the life of the product. An inspection of the clinical investigation sites by a competent authority may be required as part of the regulatory approval procedure. As a condition of marketing approval, the regulatory agency may require post-marketing surveillance to monitor adverse effects, or other additional studies as deemed appropriate. After approval for the initial indication, further clinical studies are usually necessary to gain approval for additional indications. The terms of any approval, including labeling content, may be more restrictive than expected and could affect product marketability.
Holders of an approved NDA are required to report certain adverse reactions and production problems, if any, to the FDA, and to comply with certain requirements concerning advertising and promotional labeling for their products. Moreover, quality control and manufacturing procedures must continue to conform to cGMP after approval, and the FDA periodically inspects manufacturing facilities to assess cGMP compliance. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain compliance with cGMP and other aspects of regulatory compliance. We expect to continue to rely upon third-party manufacturers to produce commercial supplies of any products which are approved for marketing. We cannot be sure that those manufacturers will remain in compliance with applicable regulations, or that future FDA inspections will not identify compliance issues at the facilities of our contract manufacturers that may disrupt production or distribution, or require substantial resources to correct.
Any of our future products approved by FDA will likely be purchased principally by healthcare providers that typically bill various third-party payors, such as governmental programs (e.g., Medicare and Medicaid), private insurance plans and managed care plans, for the healthcare products and services provided to their patients. The ability of customers to obtain appropriate reimbursement for the products and services they provide is crucial to the success of new drug and biologic products. The availability of reimbursement affects which products customers purchase and the prices they are willing to pay. Reimbursement varies from country to
61
country and can significantly impact the acceptance of new products. Even if we were to develop a promising new product, we may find limited demand for the product unless reimbursement approval is obtained from private and governmental third-party payors.
If FDA approves any of our future products and reimbursement for those products is approved by any federal or state healthcare programs, then we will be subject to federal and state laws, such as the Federal False Claims Act, state false claims acts, the illegal remuneration provisions of the Social Security Act, the federal anti-kickback laws, and state anti-kickback laws that govern financial and other arrangements among drug manufacturers and developers and the physicians and other practitioners or facilities that purchase or prescribe products. Among other things, these laws prohibit kickbacks, bribes and rebates, as well as other direct and indirect payments that are intended to induce the use or prescription of medical products or services payable by any federal or state healthcare program, and prohibit presenting a false or misleading claim for payment under a federal or state program. Possible sanctions for violation of any of these restrictions or prohibitions include loss of eligibility to participate in federal and state reimbursement programs and civil and criminal penalties. If we fail to comply, even inadvertently, with any of these requirements, we could be required to alter our operations, enter into corporate integrity, deferred prosecution or similar agreements with state or federal government agencies, and become subject to significant civil and criminal penalties.
Employees and Consultants
As of the date of this prospectus, we had four full-time employees. In addition to our employees, each of our founders serves as a consultant to the Company and consults directly with our employees and scientific staff to advance our research programs. Each of Drs. Cohen, Barzilai, Amatruda and Sinclair provide consulting services in the areas of peptide research, genetics, aging and age related diseases, drug discovery, development and commercialization and other areas relevant to our business pursuant to agreements that provide for annual compensation of $42,000 payable in monthly installments. Each agreement provides for an annual service term. The service term under the agreement with Dr. Cohen expires in September 2015. The service terms under the agreements with Drs. Barzilai, Amatruda and Sinclair expire in November 2015. We from time to time engage other subject matter experts on a consulting basis in specific areas of our research and development efforts. None of our employees are represented by a labor union or covered by a collective bargaining agreement. We have not experienced any work stoppages and we consider our relations with our employees to be good.
Our Founders and Scientific Advisors
Our founders and co-founders are widely considered to be scientific experts and thought leaders at the intersection of cellular and mitochondrial genetics and biology, the biology of aging, metabolism, and drug discovery, development and commercialization. Together, they provide scientific leadership and expertise in this field.
Founders
Pinchas Cohen, M.D. Dr. Cohen is the Dean of the Davis School of Gerontology at the University of Southern California. Dr. Cohen also holds the William and Sylvia Kugel Dean’s Chair in Gerontology and is the executive director of the Ethel Percy Andrus Gerontology Center. Dr. Cohen is a member of our board of directors and currently serves as a consultant to our company.
Nir Barzilai, M.D. Dr. Barzilai is the Director of the Institute for Aging research at Albert Einstein College of Medicine, under the auspices of which he is the principal investigator of the largest of five Nathan Shock Centers of Excellence in Biology of Aging and the Glenn Center for the Biology of Human Aging. Dr. Barzilai is a member of our board of directors and currently serves as a consultant to our company.
John Amatruda, M.D. Dr. Amatruda was formerly the Senior Vice President and Franchise Head, Diabetes and Obesity at Merck Research Laboratories. He is board certified in internal medicine, endocrinology and
62
metabolism and has approximately 20 years of experience in academic medicine, pharmaceutical discovery research and development. He has an extensive history as a principal investigator for NIH funded basic and clinical research, as well as in teaching, clinical practice. His experience includes contribution to the discovery and development of several novel candidate compounds, INDs, translational studies, development programs and five new drug approvals. Dr. Amatruda currently serves as a consultant to our company.
David Sinclair, Ph.D. Dr. Sinclair is a Professor in the Department of Genetics at Harvard Medical School and a co-joint Professor in the Department of Physiology and Pharmacology at the University of New South Wales. He is the co-Director of the Paul F. Glenn Laboratories for the Biological Mechanisms of Aging and a Senior Scholar of the Ellison Medical Foundation. He is also co-founder of both Sirtris Pharmaceuticals (NASDAQ: SIRT) and Genocea Biosciences. His laboratory at Harvard is currently focused on slowing diseases of aging in mammals using genetic and pharmacological means. Dr. Sinclair currently serves as a consultant to our company.
Scientific Advisors
In addition to the expertise of our founders, we have assembled a scientific advisory board that includes renowned experts in cardiology, diabetes, drug discovery and peptide. These advisors work in close collaboration with our founders and scientists to identify new research directions and accelerate our target validation and drug discovery programs.
| Name |
Advisory Focus |
Primary Affiliation | ||
| Amir Lerman, M.D. |
Cardiology | Mayo Clinic | ||
| C. Ronald Kahn, M.D. |
Diabetes | Harvard Medical School | ||
| James N. Livingston, Ph.D. |
Diabetes | Independent Consultant | ||
| Paul Aisen, M.D. |
Alzheimer’s disease | University of California, San Diego | ||
Legal Proceedings
We may from time to time be party to litigation and subject to claims incident to the ordinary course of business. As we grow and gain prominence in the marketplace we may become party to an increasing number of litigation matters and claims. The outcome of litigation and claims cannot be predicted with certainty, and the resolution of these matters could materially affect our future results of operations, cash flows or financial position. We are not currently a party to any legal proceedings.
Facilities
We lease laboratory space within an approximately 5,564 square foot shared laboratory facility in Pasadena, California on a month to month basis. We believe our facilities are adequate for our current needs.
63
Executive Officers and Directors
Below are the names, ages and positions held with us of our executive officers and directors as of the date of this prospectus.
| Name and State of Residence |
Age | Position(s)/Principal Occupation* | ||
| Executive Officers: |
||||
| Jon Stern California, USA |
59 | Chief Executive Officer and Director | ||
| Jeffrey F. Biunno New Jersey, USA |
48 | Chief Financial Officer, Secretary and Treasurer | ||
| Kenneth Cundy California, USA |
55 | Chief Scientific Officer | ||
| Directors: |
||||
| Albion Fitzgerald New Jersey, USA |
66 | Chairman of the Board of Directors | ||
| Nir Barzilai New York, USA |
58 | Director | ||
| Pinchas Cohen California, USA |
57 | Director | ||
| Marc E. Goldberg Massachusetts, USA |
57 | Director | ||
| * | The principal occupations of our non-employee directors are described in their respective biographical details below. |
We have entered into a standard form of Proprietary Information and Inventions Assignment Agreement with each of our executive officers. These agreements include covenants prohibiting the employee party from disclosing or using our confidential information. None of our non-employee directors are a party to any non-competition or non-disclosure agreement with us, except that Drs. Barzilai and Cohen have agreed to certain non-disclosure obligations and restrictions on use of our proprietary information pursuant to consulting agreements.
Executive Officers
Jon Stern served in senior strategic roles with our company from August 2012 until he was appointed as our chief executive officer effective in October 2013. He was appointed to our board of directors in May 2014. From 2009 to 2011 Mr. Stern served as chief operating officer of The Key Worldwide, a provider of college admissions preparation and coaching services for aspiring students and their families in the U.S. and Asia. From 2006 to 2008, Mr. Stern served as executive vice president of Integrated China Media, a Guangzhou, China-based provider of digital entertainment content for the Chinese youth market. From 2003 to 2008, Mr. Stern was a partner in Pacific Arts Group, a publisher and collection of Chinese Contemporary Fine Art. Mr. Stern founded Digital Sparx in 1999, a distributor of digital entertainment content to movie-goers and served as president and chief executive officer of that company until 2002. In 1986 Mr. Stern founded Cine Coasters, Inc., a developer and distributor of sports stadium and movie theatre products and accessories, and served as its chief executive officer until its sale to a division of Liberty Media in 1998. Mr. Stern holds an MBA from the Marshall School of Business at the University of Southern California and a B.S. in Business Administration from The University of California, Berkeley. Our board of directors believes that Mr. Stern’s substantial experience as an entrepreneur and senior executive of growth stage companies as well as established businesses and his familiarity with the day-to-day operations of our business make him a valuable contributor to our board of directors. Mr. Stern is a full-time employee of our company.
64
Kenneth Cundy joined our company as chief scientific officer in November, 2014. From December, 2012 to November, 2014, Dr. Cundy served as the chief scientific officer for XenoPort, Inc., a biopharmaceutical company focused on the development of product candidates for the potential treatment of neurological disorders. He served XenoPort, Inc. as senior vice president of preclinical and clinical sciences from 2011 to 2012, as its vice president of preclinical development from 2004 to 2011, and as its vice president of biopharmaceutics from 2000 to 2004. From 1992 to 2000, Dr. Cundy was senior director of biopharmaceutics at Gilead Sciences, Inc. Prior to Gilead Sciences, from 1988 to 1992 Dr. Cundy was principal research investigator at Sterling Drug, a pharmaceutical division of Eastman Kodak Company. He received a B.S. in pharmacy from the University of Manchester and was registered as a pharmacist in the UK. He received a Ph.D. in pharmaceutical sciences from the University of Kentucky and postdoctoral training in biochemistry at the University of California, Berkeley. Dr. Cundy is a full-time employee of our company.
Jeffrey F. Biunno joined our company in October 2013 as chief financial officer and was appointed secretary and treasurer in September 2014. Prior to joining Cohbar, Mr. Biunno served as chief financial officer, secretary and treasurer of ManageIQ, Inc., a provider of global cloud IT systems management solutions, from March, 2012 until its acquisition by Red Hat, Inc. in December 2012. From February 2009 until March 2012 Mr. Biunno served as vice president and worldwide controller of Dialogic Inc., a provider of mobile telecommunications network software and hardware enterprise solutions then listed on NASDAQ. Mr. Biunno founded Scalable Financial Solutions, LLC, a financial consulting firm, and operated it from March 2008 to January 2009. From February 2005 to March 2008, Mr. Biunno worked at Geller & Company, a financial services consulting firm. From 1997 to 2004 Mr. Biunno served as vice president and corporate controller of Novadigm, Inc. (NASDAQ: NVDM), an international provider of IT systems management solutions to Fortune 500 companies and government agencies. Mr. Biunno received a B.S. in accounting and an MBA in finance from Montclair State University. Mr. Biunno is a certified public accountant and a chartered global management accountant. Mr. Biunno is a full-time employee of our company.
Directors:
Albion J. Fitzgerald has served as a member of our board of directors since May 2014 and was appointed as chairman in July 2014. Mr. Fitzgerald previously served as chief executive officer and chairman of the board of directors of ManageIQ, Inc., a provider of global cloud IT systems management solutions. Mr. Fitzgerald was appointed as a director of ManageIQ in 2007, and served as strategic consultant to the company from 2007 until April 2012, and as chief executive officer and chairman of the board of directors from April 2012 until ManageIQ, Inc. was acquired by Red Hat, Inc. in December 2012. In 1992 Mr. Fitzgerald co-founded Novadigm, Inc. (NASDAQ: NVDM), an international provider of IT systems management solutions to Fortune 500 companies and government agencies with customers in 26 countries. He served as its chairman and chief technology officer from 1992 and chief executive officer from 1995 until its acquisition by Hewlett Packard in 2004. Prior to Novadigm, Mr. Fitzgerald founded and served as chief executive officer of Telemetrix, Inc., a provider of enterprise IT systems and network management solutions. From 1980 to 1989, Mr. Fitzgerald was a strategic technology consultant to New York University responsible for architecting, building and managing the university’s computer center and campus-wide multi-media network. Mr. Fitzgerald began his technology career at IBM in 1966. Our board of directors believes that Mr. Fitzgerald’s extensive experience in founding, funding and building emerging technology companies, the depth of his technology and business expertise, and his relevant experience as a director and officer of a publicly-traded enterprise software company make him a valuable contributor to our board of directors.
Dr. Nir Barzilai co-founded our company in 2007 and has served as a member of our board of directors since our conversion to a Delaware corporation in 2009. Dr. Barzilai is the director of the Institute for Aging Research at the Albert Einstein College of Medicine of Yeshiva University, where he also holds the Ingeborg and Ira Leon Rennert Chair of Aging Research, is a professor in the Departments of Medicine and Genetics and a member of the Diabetes Research Center. Dr. Barzilai is also director of the Paul F. Glenn Center for the Biology of Human Aging Research and of the National Institutes of Health’s (NIH) Nathan Shock Centers of Excellence in the Basic Biology
65
of Aging. Dr. Barzilai has received numerous awards, including the Beeson Fellow for Aging Research, the Ellison Medical Foundation Senior Scholar in Aging Award, the Paul F. Glenn Foundation Award, the NIA Nathan Shock Award, and the 2010 Irving S. Wright Award of Distinction in Aging Research. Dr. Barzilai’s leadership in gerontology research and his experience overseeing numerous research programs related to diseases of aging and mitochondrial biology makes him an important contributor to our board of directors.
Dr. Pinchas Cohen co-founded our company in 2007 and has served as a member of our board of directors since our conversion to a Delaware corporation in 2009. He served as our President from 2009 until 2013. Since April 2012, Dr. Cohen has served as dean of the Davis School of Gerontology at the University of Southern California. He holds the William and Sylvia Kugel Dean’s Chair in Gerontology and acts as executive director of the Ethel Percy Andrus Gerontology Center. Dr. Cohen was affiliated with the University of California, Los Angeles School of Medicine, where he was a member of the faculty until 2012. At the UCLA Mattel Children’s Hospital Dr. Cohen served as director of endocrine/diabetes research and training (from 1999 until 2012), chief of endocrinology and diabetes (from 2001 until 2012) and as vice chair of research (from 2011 until 2012). Dr. Cohen was also co-director of the UCSD-UCLA Diabetes and Endocrinology Research Center from 2007 until 2012. Dr. Cohen has received several awards for his work in the field of aging, including a National Institute of Aging EUREKA Award, the National Institutes of Health Director’s Transformative Research Award and the Glenn Award for Research in Biological Mechanisms of Aging. He serves on the boards of several professional journals and societies, including the American Federation for Aging Research and the Growth Hormone Research Society. Our board of directors believes that Dr. Cohen’s leadership in gerontology research and his experience overseeing numerous research programs related to diseases of aging and mitochondrial biology makes him an important contributor to our board of directors.
Marc E. Goldberg joined our board of directors in November, 2014. Mr. Goldberg is a Managing Director at BioVentures Investors, a life science focused venture and private equity investment firm that he co-founded in 1997. Prior to founding BioVentures, Mr. Goldberg served as the president and chief executive officer of the Massachusetts Biotechnology Research Institute from 1991 to 1997. From 1987 to 1991, Mr. Goldberg was the vice president of finance and corporate development, chief financial officer, and treasurer at Safer, Inc., a developer and manufacturer of biopesticides and related products. Prior to joining Safer, he served as the manager of business development at Genetics Institute. Mr. Goldberg was also Founding President of the Massachusetts Biotechnology Council and served four terms as its president and as a director from 1985 to 1997. He is currently a member of the Harvard Medical School Neuroscience Advisory Committee and he previously served as a member of the Beth Israel Deaconess Medical Center Research Advisory Committee of the board of directors. From 2002 to 2014 Mr. Goldberg served on the Board of Directors of Enanta Pharmaceuticals, Inc. (NASDAQ:ENTA), a biotechnology company engaged in developing small molecule drugs. He has also served as a director of a number of private companies within the biotechnology, pharmaceutical and medical technology industries. Mr. Goldberg received an A.B. from Harvard College, a J.D. from Harvard Law School and an M.B.A. from Harvard Business School. Our board of directors believes that Mr. Goldberg’s extensive experience in growing and financing emerging biotechnology and pharmaceutical companies, as well as the depth of his business and financial expertise, make him a valuable contributor to our board of directors.
Biographical information related to our director Jon Stern is included under the heading “Executive Officers.”
Each of our directors serves until the next annual meeting of our stockholders and until his successor is duly elected and qualified, subject to his earlier resignation or removal.
Cease Trade Orders, Bankruptcies and Penalties and Sanctions
None of our directors, executive officers or control persons is, or within the ten years prior to the date of this prospectus has been, (a) a director, chief executive officer or chief financial officer of any issuer (including us) that, (i) was subject to an order that was issued while that person was acting in the capacity as director, chief
66
executive officer or chief financial officer, or (ii) was subject to an order that was issued after that person ceased to be a director, chief executive officer or chief financial officer and which resulted from an event that occurred while that person was acting in the capacity as director, chief executive officer or chief financial officer; or (b) a director or executive officer of any company (including us) that, while that person was acting in that capacity, or within a year of that person ceasing to act in that capacity, became bankrupt, made a proposal under any legislation relating to bankruptcy or insolvency or was subject to or instituted any proceedings, arrangement or compromise with creditors or had a receiver, receiver manager or trustee appointed to hold its assets. For the purposes of this paragraph, “order” means a cease trade order, an order similar to a cease trade order or an order that denied the relevant company access to any exemption under securities legislation, in each case that was in effect for a period of more than 30 consecutive days.
None of our directors, officers or control persons has been subject to any penalties or sanctions imposed by a court relating to securities legislation or by a securities regulatory authority or has entered into a settlement agreement with a securities regulatory authority or has been subject to any other penalties or sanctions imposed by a court or regulatory body which would be important to a reasonable investor making an investment decision.
None of our directors, officers or control persons (or a personal holding company of any such person) is, or within the ten years prior to the date of this prospectus has become, bankrupt, made a proposal under any legislation relating to bankruptcy or insolvency or has been subject to or instituted any proceedings, arrangement or compromise with creditors, or had a receiver, receiver manager or trustee appointed to hold his assets.
Conflicts of Interest
Our directors and officers are or may be stockholders of other public companies, and may in the future become directors or officers of other public companies. Accordingly, conflicts of interest may arise between such persons’ duties as directors and officers of our company and their positions as directors and stockholders of such other companies. All such possible conflicts are required to be disclosed in accordance with the requirements of applicable corporate law and the directors and officers are required to act in accordance with the obligations imposed on them by law. See “Certain Related Party Transactions – Policies and Procedures for Related Party Transactions” below.
Board Composition
The number of members of our board of directors is currently fixed at five members, and may be modified from time to time by resolution of our board of directors. Our stockholders elect our board of directors to govern our business and affairs. Our board of directors selects our senior management team, which is charged with conducting our business. Having selected our senior management team, our board of directors acts as an advisor to senior management, monitors their performance and reviews our strategies, financial objectives and operating plans. It also plans for management succession of our Chief Executive Officer, as well as other senior management positions, and oversees our compliance efforts.
Director Independence
Our board of directors has determined to evaluate the independence of our directors by reference to NASDAQ Rule 5605(a)(2) of the NASDAQ Stock Market LLC (NASDAQ) and National Instrument 52-110 – Audit Committees (NI 52-110). Our board of directors has determined that Messrs. Fitzgerald and Goldberg are independent under these standards. Our board of directors may meet independently of management as required. Although they are permitted to do so, the independent directors have not held regularly scheduled meetings at which non-independent directors and members of management are not in attendance.
67
Board Committees
Our board of directors has established an audit committee, a compensation committee and a governance and nominating committee, each to be effective upon the completion of the offering. Our audit committee charter is included as an exhibit hereto, and will be available, along with each other committee’s charter, on our website at www.cohbar.com following completion of the offering.
Audit Committee
Effective upon the completion of this offering, our audit committee will consist of Albion J. Fitzgerald, Marc E. Goldberg and Nir Barzilai. Mr. Goldberg will serve as chair of our audit committee. Our board of directors has evaluated the independence and qualification of our directors to serve on our Audit Committee based on applicable rules of NASDAQ and the SEC rules and NI 52-110, and has determined that each of Messrs. Fitzgerald and Goldberg will be an independent director as defined by such standards. Our board of directors determined that each of the committee members will meet the requirements of financial literacy under SEC rules and regulations and by NI 52-110, and that Mr. Goldberg meets the requirements for designation as an “audit committee financial expert”, as defined under SEC rules.
Rule 10A-3 of the Securities Exchange Act of 1934, as amended, (Exchange Act) requires that the audit committees of public companies whose securities are traded on certain United States securities exchanges be composed entirely of independent directors, subject to certain transitional accommodations afforded in connection with an initial public offering. The rules of the TSX-V require that the Company’s audit committee be composed of at least three members, at least two of whom must be independent. The composition of our audit committee upon completion of this offering will comply with the requirements of the TSX-V. Although we may in the future seek to compose our audit committee entirely of independent directors, we do not believe that the inclusion of Dr. Barzilai as a member of our audit committee will materially adversely affect the ability of our audit committee to act independently, nor have we determined that the composition of our audit committee presents material risks related to the reporting of our financial statements. The Company will be a “venture issuer” as defined in NI 52-110 and is relying on the exemption in section 6.1 of NI 52-110 relating to Parts 3 (Composition of Audit Committee) and 5 (Reporting Obligations).
Each member of the Company’s audit committee has experience and/or an educational background that is relevant to the performance of his duties as an audit committee member. Mr. Goldberg holds J.D. and M.B.A. degrees, and has gained experience relevant to performance of his audit committee duties in high level executive roles, including service as chief financial officer of a biopesticides manufacturer, and through service as a director and member of the audit committee of a publicly traded biotechnology company. Mr. Fitzgerald has gained experience relevant to performance of his audit committee duties in high level executive roles, including service as director and chief executive officer of both private and publicly-traded enterprise software companies. Dr. Barzilai has gained experience relevant to performance of his audit committee duties through his roles as an academic and laboratory administrator and in his capacity as a private investor.
The audit committee will be responsible for, among other things:
| • | selecting and hiring our independent registered public accountants, and approving the audit and non-audit services to be performed by such firm; |
| • | evaluating the qualifications, performance and independence of our independent registered public accountants; |
| • | monitoring the integrity of our financial statements and our compliance with legal and regulatory requirements as they relate to financial statements or accounting matters; |
| • | reviewing the adequacy and effectiveness of our internal control policies and procedures; |
| • | discussing the scope and results of the audit and interim reviews as well as operating results with management and the independent registered public accountants; |
68
| • | preparing the audit committee report that the SEC requires in our annual proxy statement; and |
| • | reviewing the fees paid by us to our independent registered public accountants in respect of audit and non-audit services on an annual basis. |
Our board of directors has adopted, effective upon completion of the offering, a written charter for our audit committee, which is attached as an exhibit hereto, and will be available on our website following completion of the offering.
Compensation Committee
Effective upon completion of the offering our board of directors established a compensation committee consisting of Messrs. Fitzgerald and Goldberg.
Our compensation committee will be responsible for, among other things:
| • | reviewing and approving compensation of our executive officers, including annual base salary, annual incentive bonuses, specific goals, equity compensation, employment agreements, severance and change in control arrangements, and any other benefits, compensations or arrangements; |
| • | reviewing and recommending compensation goals, bonus and stock compensation criteria for our employees; |
| • | preparing the compensation committee report required by the SEC to be included in our annual proxy statement; and |
| • | administering, reviewing and making recommendations with respect to our equity compensation plans. |
Our board of directors has adopted effective upon completion of the offering, a written charter for our compensation committee, which will be available on our website at such time.
Governance and Nominating Committee
Effective upon completion of the offering our board of directors established a governance and nominating committee consisting of Mr. Goldberg, Mr. Fitzgerald and Dr. Cohen.
Our governance and nominating committee will be responsible for, among other things:
| • | assisting our board of directors in identifying, interviewing and recruiting prospective director nominees; |
| • | recommending director nominees; |
| • | establishing and reviewing on an annual basis a process for identifying and evaluating nominees for our board of directors; |
| • | annually evaluating and reporting to the our board of directors on the performance and effectiveness of the board of directors; |
| • | recommending members for each board committee of our board of directors; and |
| • | annually presenting a list of individuals recommended for nomination for election to our board of directors at the annual meeting of our stockholders. |
Our board of directors has adopted, effective upon completion of the offering, a written charter for our governance and nominating committee, which will be available on our website at such time.
69
Code of Ethics and Business Conduct
The board of directors encourages and promotes a culture of ethical business conduct through communication and supervision as part of their overall stewardship responsibility. Our board of directors adopted a code of ethics and business conduct applicable to all of our employees, including our executive officers and directors, and those employees responsible for financial reporting. Our code of ethics and business conduct establishes procedures that allow our directors, officers and employees to confidentially submit their concerns regarding questionable ethical, moral, accounting or auditing matters, without fear of retaliation. The code of business conduct and ethics will be available on our website upon completion of the offering. We expect that, to the extent required by law, any amendments to the code, or any waivers of its requirements, will be disclosed on our website and in mandatory filings.
Canadian Governance Matters and Disclosure
Generally
The Canadian Securities Administrators have published National Policy 58-201 – Corporate Governance Guidelines. This instrument sets out a series of non-binding guidelines and requirements for effective corporate governance and in this prospectus we refer to them collectively as the “Guidelines.” The Guidelines address matters such as the constitution and independence of corporate boards, the functions to be performed by boards and their committees and the effectiveness and education of board members.
Independence
Our board of directors has determined that each of our directors other than Jon Stern, Pinchas Cohen and Nir Barzilai is independent for the purpose of National Instrument 58-101 – Disclosure of Corporate Governance Practices.
None of our directors presently hold directorships with other reporting issuers.
Orientation and Continuing Education
Our board of directors is responsible for the orientation and education of new members of the board and all new directors are provided with copies of our policies. Prior to joining the board, each new director will meet with our chairman and chief executive officer. Our chairman and chief executive officer are responsible for outlining our business and prospects, both positive and negative, with a view to ensuring that the new director is properly informed to commence their duties as a director. Each new director is also given the opportunity to meet with our auditors and counsel. As part of its annual self-assessment process, our board of directors determines whether any additional education and training is required for board members.
Nomination of Directors
Historically, because of our size and stage of development and the limited number of directors, the entire board of directors has taken responsibility for nominating new directors and assessing current directors. As of the closing of this offering, nominees for election to our board of directors will be identified, interviewed and recruited by our governance and nominating committee. For additional information about our governance and nominating committee, see “Management – Board Committees – Governance and Nominating Committee” above.
Compensation
Historically, because of our size and stage of development and the limited number of directors, the compensation of our executive officers and directors was determined by our board of directors as a whole. As of
70
the closing of this offering, our compensation committee will be responsible for reviewing and approving the compensation of our executive officers and directors and for reviewing and recommending compensation goals, bonus and stock compensation criteria for our employees. For additional information about our compensation committee, see “Management – Board Committees – Compensation Committee” above.
Audit Fees
No audit, audit-related, tax or other fees of our auditors, Marcum LLP, were paid, billed or accrued by the Company during fiscal 2012 or 2013. Subsequent to December 31, 2013, we were billed approximately $40,000 in audit fees for the years ended December 31, 2013, 2012 and 2011.
Assessment
Historically, because of our size and stage of development and the limited number of directors, our board of directors considered a formal assessment process to be unnecessary and evaluated its own effectiveness on an ad hoc basis. As of the closing of this offering, our governance and nominating committee will be responsible for annually evaluating and reporting to our board of directors on the performance and effectiveness of the board as a whole and its committees. For additional information about our governance and nominating committee, see “Management – Board Committees – Governance and Nominating Committee” above.
Compensation of Directors
The following table sets forth information concerning the compensation for the fiscal year ended December 31, 2013 of our directors who are not also named executive officers:
DIRECTOR COMPENSATION – YEAR ENDED DECEMBER 31, 2013
| Name |
Fees Earned or Paid in Cash($) |
Option Awards($) |
All Other Compensation(1) |
Total | ||||||||||||
| Nir Barzilai |
— | — | $ | 12,000 | $ | 12,000 | ||||||||||
| Pinchas Cohen |
— | — | $ | 12,000 | $ | 12,000 | ||||||||||
| Albion J. Fitzgerald(2) |
— | — | — | — | ||||||||||||
| Marc E. Goldberg(2) |
— | — | — | — | ||||||||||||
| (1) | Represents fees paid to Drs. Barzilai and Cohen for consulting services. |
| (2) | Messrs. Fitzgerald and Goldberg were appointed to our board of directors in May, 2014 and November, 2014, respectively. |
No member of our board of directors received compensation for their service as a director during the fiscal year ended December 31, 2013. On November 20, 2014, the board of directors approved compensation for Albion J. Fitzgerald and Marc E. Goldberg for their services to the Company as directors. Messrs. Goldberg and Fitzgerald are each entitled to an annual cash retainer of $15,000 and were each granted options to purchase up to 250,000 shares of common stock. The stock options have an exercise price of $0.73 and vest in a series of forty-eight (48) equal successive monthly installments commencing on November 3, 2014, such that all shares subject to the options shall be vested and exercisable on the fourth (4th) anniversary of the that date, subject to the continuous service of that director through each such vesting date. Vesting of the options is subject to acceleration under certain circumstances, including a change of control. All directors are entitled to reimbursement of ordinary expenses incurred in connection with attendance at meetings of our board of directors.
71
Compensation of our executive officers is designed to provide compensation that is competitive, as well as consistent with our early stage of development. Our board recognizes the need to provide a compensation package that will attract and retain qualified and experienced executives as well as align the compensation level of each executive to that executive’s level of responsibility. Our compensation arrangements are not divided formally into long-term and short-term plans; however as a general matter our compensation programs may include shorter term incentive compensation in the form of annual cash bonuses payable on achievement of individual and company performance goals that may be established from time to time, and longer term incentives through the grant of stock options with vesting over a period of years. We do not maintain a pension plan that provides for cash or other payments upon retirement.
The compensation of our executives has been determined by our board. Upon completion of the offering we expect that our newly established compensation committee will oversee the design and administration of our executive compensation programs and provide recommendations to our board, including in relation to establishment of annual performance objectives for our executive officers.
Our board has not undertaken a formal evaluation of the implications of the risks associated with our compensation policies and practices. However, risk management is a consideration of our board when implementing our compensation program and the board does not believe that our compensation programs encourage unnecessary or inappropriate risk taking, including risks that are likely to have a material adverse effect on the company.
Summary Compensation Table
The following table provides certain summary information concerning the compensation awarded to, earned by or paid to (i) all persons serving as our Principal Executive Officer (PEO), and (ii) our two most highly compensated executive officers other than our PEO, who were serving as executive officers at the end of the last completed fiscal year (herein referred to as the “named executive officers”) for the fiscal years ended December 31, 2013 and 2012.
| Name and Principal Position |
Fiscal Year |
Salary ($) |
Bonus ($) |
Stock Awards ($) |
Option Awards ($) |
All Other ($) |
Total ($) |
|||||||||||||||||||||
| Jon Stern(1) |
2013 | $ | 50,000 | — | — | — | $ | 118,280 | $ | 168,280 | ||||||||||||||||||
| Chief Executive Officer and Director |
2012 | — | — | — | — | — | — | |||||||||||||||||||||
| Jeffrey F. Biunno(2) |
2013 | $ | 30,769 | — | — | — | — | $ | 30,769 | |||||||||||||||||||
| Chief Financial Officer, Secretary and Treasurer |
2012 | — | — | — | — | — | — | |||||||||||||||||||||
| Mark A. Rampy(3) |
2013 | $ | 110,965 | — | — | — | — | $ | 110,965 | |||||||||||||||||||
| Former Chief Executive Officer and Director |
2012 | $ | 276,990 | — | — | $ | 58,451 | — | $ | 335,441 | ||||||||||||||||||
| (1) | Mr. Stern served as our Chief Strategy Officer from May 1, 2013, until his appointment as our Chief Executive Officer effective October 13, 2013. Mr. Stern deferred compensation for his services performed through April 11, 2014. Pursuant to a letter agreement dated April 11, 2014, Mr. Stern received in consideration of his services through such date (i) a cash payment of $71,042 and (ii) a warrant to purchase 797,075 shares of our common stock at an exercise price equal to $0.26 per share. Mr. Stern’s salary for 2013 reported above reflects the portion of such cash payment attributable to his service through December 31, 2013. The amount reported as other compensation for 2013 reflects that portion of the warrant’s grant date fair value that is attributable to Mr. Stern’s service through December 31, 2013. |
72
| (2) | Mr. Biunno was appointed as our Chief Financial officer in October 2013. |
| (3) | Dr. Rampy served as our Chief Executive Officer until May 2013. The amount reported in the Option Awards column represents the grant date fair value of a stock option award granted to Dr. Rampy in 2012, as computed in accordance with FASB ASC 718. The assumptions used in calculating the grant date fair value of the stock options reported in the Option Awards column are set forth in Note 3 to our audited financial statements for the years ended December 31, 2013, 2012 and 2011 included elsewhere in the registration statement of which this prospectus forms a part. The amounts reported in this column excludes the impact of estimated forfeitures related to service-based vesting conditions, reflect the accounting cost for these stock options. All stock options held by Dr. Rampy expired unexercised following his separation from service as our Chief Executive Officer. |
Executive Employment Agreements
We entered into an Executive Employment Agreement with Mr. Stern, dated April 11, 2014, which sets forth conditions of Mr. Stern’s at-will employment with our company. Mr. Stern also executed the Company’s standard form of Proprietary Information and Inventions Assignment Agreement. Mr. Stern’s current base salary is $250,000 annually, and he is eligible under the agreement for an annual bonus of up to 35% of his annual salary, payable at the discretion of the board of directors upon achievement of performance targets established by the board of directors from time to time. The Executive Employment Agreement entitles Mr. Stern to certain severance payments and other benefits if his employment is terminated by us without cause, or upon his resignation for good reason as defined in the Executive Employment Agreement. Upon any such termination of Mr. Stern’s employment he would be entitled to a severance payment equal to fifty percent (50%) of his then current base salary, and reimbursement for any COBRA coverage elected by Mr. Stern for himself and the members of his immediate family for a period of six months following such termination. Additionally, any options that would have vested during the twelve (12) month period immediately following his termination date would vest and become exercisable immediately. On April 9, 2014 Mr. Stern was granted options to purchase 478,245 shares of our common stock at an exercise price of $0.26. The options vest and become exercisable over 36 equal monthly installments commencing May 1, 2013. Pursuant to the Stock Option Agreement applicable to Mr. Stern’s award and our 2011 Equity Incentive Plan, the options immediately vest and become exercisable under certain circumstances, including a change of control. See “2011 Equity Incentive Plan – Change of Control Provisions”.
We entered into an Executive Employment Agreement with Jeffrey F. Biunno, dated November 27, 2013, which sets forth certain conditions of Mr. Biunno’s at-will employment with the Company. Mr. Biunno also executed the Company’s standard form of Proprietary Information and Inventions Assignment Agreement. The Executive Employment Agreement entitles Mr. Biunno to a base salary of $200,000 annually, and eligibility for an annual bonus of up to $50,000 payable at the discretion of the board of directors upon achievement of performance targets established by the board of directors. Mr. Biunno is entitled to a severance payment in an aggregate gross amount equal to fifty percent (50%) of his then current base salary if his employment is terminated by us without cause. On April 9, 2014 Mr. Biunno was granted options to purchase 364,377 shares of our common stock at an exercise price of $0.26 per share. 236,845 shares subject to the option grant vest based solely on Mr. Biunno’s continued service. One quarter (1/4) of such shares vest and become exercisable on October 31, 2014 and the remaining shares subject to the option will vest in 36 monthly installments thereafter. 127,532 shares subject to the option grant vest based both on Mr. Biunno’s continued service through the relevant vesting date and completion of our initial public offering. Assuming completion of our initial public offering, one quarter (1/4) of shares subject to the option become vested on October 31, 2014, and the remaining shares subject to the option will become exercisable in 36 monthly installments thereafter. Pursuant to the Stock Option Agreement applicable to Mr. Biunno’s award and our 2011 Equity Incentive Plan, the options immediately vest and become exercisable under certain circumstances, including a change of control. See “2011 Equity Incentive Plan – Change of Control Provisions”.
73
We entered into an Executive Employment Agreement with Kenneth Cundy, dated November 17, 2014, which sets forth certain conditions of Dr. Cundy’s at-will employment with the Company as the Company’s Chief Scientific Officer. Dr. Cundy also executed the Company’s standard form of Proprietary Information and Inventions Assignment Agreement. The Executive Employment Agreement entitles Dr. Cundy to a base salary of $300,000 annually, and eligibility for an annual bonus of up to $75,000 payable at the discretion of the board of directors upon achievement of performance targets established by the board of directors. The Executive Employment Agreement entitles Dr. Cundy to certain severance payments and other benefits if his employment is terminated by us without cause, or upon his resignation for good reason as defined in the Executive Employment Agreement. Upon any such termination of Dr. Cundy’s employment he would be entitled to a severance payment equal to fifty percent (50%) of his then current base salary, and reimbursement for any COBRA coverage elected by Dr. Cundy for himself and the members of his immediate family for a period of six months following such termination. Additionally, any options that would have vested during the twelve (12) month period immediately following his termination date would vest and become exercisable immediately. Dr. Cundy was granted options to purchase 860,000 shares of our common stock at an exercise price of $0.73 per share. One quarter (1/4) of those options will vest and become exercisable on the first (1st) anniversary of Dr. Cundy’s first date of employment and the remaining shares subject to the option will vest in thirty-six (36) equal monthly installments thereafter. Pursuant to the Stock Option Agreement applicable to Dr. Cundy’s award and our 2011 Equity Incentive Plan, the options immediately vest and become exercisable under certain circumstances, including a change of control. See “2011 Equity Incentive Plan – Change of Control Provisions”.
2011 Equity Incentive Plan
Our 2011 Equity Incentive Plan, as amended from time to time, which we refer to as our “2011 Plan”, was originally adopted by our board of directors and was approved by our stockholders on January 3, 2012. The 2011 Plan provides for the award of incentive stock options, nonstatutory stock options, stock appreciation rights, restricted stock awards and restricted stock unit awards. Prior to completion of the offering our board of directors intends to adopt an Amendment and Restatement of the 2011 Plan, which we refer to as the “Restatement”. The Restatement is expected to be approved by our stockholders prior to, but conditional upon, the completion of the offering. Except where indicated, the description below refers generally to the 2011 Plan as currently in effect and as amended by the Restatement.
Shares Available for Awards. As of the date of this prospectus, we had an aggregate of 2,616,041 shares of our common stock reserved for issuance under the 2011 Plan. As of the date of this prospectus, we had not issued any shares of our common stock upon the exercise of options, restricted stock purchase rights and restricted stock granted under the 2011 Plan and there were outstanding options to purchase 2,609,811 shares of our common stock and 6,230 shares of our common stock remained available for issuance under the 2011 Plan. Upon the effectiveness of the Restatement all outstanding awards will become subject to the terms of the Restatement, which will increase the total number of shares authorized under the 2011 Plan to a number of shares of our common stock equal to 20% of our outstanding common stock upon completion of the offering, the maximum permitted under the rules of the TSX-V.
Eligibility. Awards may be granted to our employees, directors, and consultants, except that only our employees are eligible to receive incentive stock options.
Administration. The 2011 Plan provides that it shall be administered by our board of directors or a committee appointed by our board of directors, which shall be constituted to comply with laws, regulations or the rules of any stock exchange or quotation system on which shares of our common stock are then listed or quoted related to the administration of stock option plans. Our board of directors has acted as the administrator of our 2011 Plan. Our board of directors has the full and final power and authority to determine the terms of awards under the 2011 Plan, including designating the persons who will receive awards, the types of awards that will be granted, the fair market value of the shares of common stock underlying each option granted to each participant if the shares are not listed on an exchange or regularly quoted by a recognized securities dealer, and the terms,
74
conditions and restrictions applicable to each award and the underlying shares. Awards under the 2011 Plan are evidenced by award agreements subject to approval by our board of directors.
Stock Options. Under our 2011 Plan, our board of directors may grant employee participants incentive stock options, which qualify for special tax treatment under U.S. tax law, and may grant nonqualified stock options to any participants. Our board of directors establishes the duration of each option at the time such option is granted, with a maximum duration of 10 years from the effective date of the grant; provided, however, that an incentive stock option granted to a participant who owns, at the time the option is granted, more than 10% of the total combined voting power of all classes of our stock may not have a term in excess of 5 years. Our board of directors also establishes any vesting requirements, including performance criteria or passage of time, which must be satisfied prior to the exercise of the options. Our board of directors also establishes the exercise price of options on the date such options are granted. Incentive stock options must have an exercise price per share that is not less than the fair market value of a share of our common stock on the grant date and, pursuant to the rules of the TSX-V, not less than the Discounted Market Price, and in the case of grants of incentive stock options to an employee who owns, at the time the option is granted, more than 10% of the total combined voting power of all classes of our stock, the exercise price may not be less than 110% of the fair value. The method of payment of the exercise price for shares being purchased pursuant to a stock option is determined by our board of directors, provided that the exercise price for options granted after effectiveness of the Restatement may be paid only by cash or check. Unless otherwise determined by our board of directors and included in the participant’s stock option agreement, after the termination of the participant’s employment or service, such participant may exercise his or her option for 90 days, except that if the termination results from the participant’s death or disability the exercise period is 12 months.
Adjustment of Shares. The number of shares issued or reserved for issuance pursuant to the 2011 Plan is subject to adjustment in order to prevent diminution or enlargement of benefits intended to be made available under the plan in the event of any dividend or other distribution, recapitalization, stock split, reverse stock split, reorganization, merger, consolidation, split-up, spin-off, combination, repurchase or exchange of our common stock, or our other securities, or other change of our corporate structure that affects our corporate structure.
Change in Control Provisions. Stock options and other awards under the Plan may be subject to acceleration of vesting and exercisability upon or after a change in control as may be provided in the agreement applicable to the option or award, or as may be provided in any other written agreement between us and the awardee, or as may be determined by the board of directors, but in the absence of such provision, no such acceleration shall occur. The plan defines a change in control as the occurrence, in a single transaction or a series of related transactions of any of the following events: (i) any person (as such term is used in Sections 13(d) and 14(d) of the Exchange Act) becomes beneficial owner (as defined in Rule 13d-3 of the Exchange Act), directly or indirectly, of our securities that represent 50% or more of the total voting power represented by all of our then-outstanding voting securities (except for any such change in beneficial ownership occurring as a result of a private financing transaction that was approved by our board of directors); (ii) a merger, consolidation or similar transaction after consummation of which our stockholders immediately prior thereto do not own, directly or indirectly, either (A) outstanding voting securities representing more than fifty percent (50%) of the combined outstanding voting power of the surviving entity in such merger, consolidation or similar transaction or (B) more than fifty percent (50%) of the combined outstanding voting power of the parent of the surviving entity in such merger, consolidation or similar transaction, in each case in substantially the same proportions as their ownership of the outstanding voting securities of the Company immediately prior to such transaction; or (iii) a sale, lease, exclusive license or other disposition of all or substantially all of our assets.
Other Terms. Unless our board of directors determines otherwise, and subject to applicable laws and exchange rules, a participant may not sell, pledge, assign, transfer or otherwise dispose of any option or restricted stock purchase right other than by will or the laws of descent and distribution, and the option or restricted stock purchase right may be exercised during the lifetime of the participant only by the participant. No shares of our common stock may be issued upon the exercise of an award unless such exercise and issuance, and the delivery to the participant of the shares of our common stock underlying such award, comply with all laws, regulations or
75
the rules of any stock exchange or quotation system on which shares of our common stock are then listed or quoted related to the administration of stock option plans.
The Restatement will include additional limits on the number of shares reserved for issuance under the 2011 Plan based on requirements of the TSX Venture Exchange, or TSX-V. The restrictions include the following:
| • | the aggregate number of shares reserved for issuance under the 2011 Plan will not be permitted to exceed 20% of the number of our issued and outstanding shares as of the date of closing of the offering, and the number of shares reserved for issuance under the 2011 Plan may not be amended without stockholder approval; |
| • | the aggregate number of options granted to any one person under the 2011 Plan in a 12 month period must not exceed five percent (5%) of the outstanding shares of common stock (on a non-diluted basis, calculated on the date the option is granted), unless the Company has obtained disinterested stockholder approval; |
| • | in the aggregate, no more than ten percent (10%) of the issued and outstanding shares of common stock (on a non-diluted basis) may be reserved at any time for insiders (as defined in the TSX-V rules) under the 2011 Plan, unless the Company has obtained disinterested stockholder approval for the 2011 Plan; |
| • | the number of options granted to insiders within any 12-month period, cannot exceed ten percent (10%) of the issued and outstanding shares of common stock, unless the Company has obtained disinterested stockholder approval for the 2011 Plan; |
| • | options shall not be granted if the exercise thereof would result in the issuance of more than two percent (2%) of the issued shares of common stock of the Company in any 12-month period to any one consultant of the Company; |
| • | options shall not be granted if the exercise thereof would result in the issuance of more than two percent (2%) of the issued shares of common stock of the Company in any 12-month period to persons engaged to provide investor relations activities; and |
| • | the number of shares of common stock subject to an option granted to any one participant shall be determined by the board of directors, but no one participant shall be granted an option to purchase a number of shares of common stock that exceeds the maximum number permitted by the rules of the TSX-V. |
Amendment and Termination. Our board of directors may amend, alter, suspend or terminate the 2011 Plan, provided that any such change that would adversely affect the holder of a previously granted award requires the holder’s written consent, and provided further that stockholder approval is required for any amendment to the extent necessary or desirable to comply with laws, regulations or the rules of any stock exchange or quotation system on which shares of our common stock are then listed or quoted related to the administration of stock option plans.
Other Benefits and Perquisites
Historically, we have not provided perquisites or other personal benefits to our executive officers. We do not have a pension plan that provides for payments to any of our executives at, following, or in connection with retirement and do not plan to establish one in the near future. We may provide perquisites or other personal benefits in limited circumstances, such as where we believe it is appropriate to assist an individual executive officer in the performance of his or her duties, to make our executive officers more efficient and effective, and for recruitment, motivation or retention purposes. We do not expect that these perquisites or other personal benefits will be a significant aspect of our executive compensation program. All future practices with respect to perquisites or other personal benefits will be approved and subject to periodic review by the Compensation Committee.
76
Outstanding Equity Awards
There were no equity awards to the named executive officers outstanding as of December 31, 2013. The following table lists all equity awards to the named executive officers outstanding as of September 30, 2014:
| Option Awards | ||||||||||||||||||||
| Grant Date(1) | Number of Securities Underlying Unexercised Options |
Option Exercise Price ($) |
Option Expiration Date |
|||||||||||||||||
| Name |
Exercisable (#) | Unexercisable (#) | ||||||||||||||||||
| Jon Stern |
4/09/2014 | (2) | 212,544 | 265,701 | 0.26 | 4/09/2024 | ||||||||||||||
| Jeffrey F. Biunno |
4/09/2014 | (3) | — | 364,377 | 0.26 | 4/09/2024 | ||||||||||||||
| (1) | All of the options identified were granted under our 2011 Equity Incentive Plan. |
| (2) | Options vest in 36 equal monthly installments beginning May 1, 2013. |
| (3) | 236,845 of these stock options vest based solely on continued service, with one quarter of such options vesting on October 31, 2014 and the remaining shares subject to the option vesting in thirty-six monthly installments thereafter. 127,532 of these stock options vest based upon continuous service and completion of our initial public offering. Assuming completion of our initial public offering, one-quarter of such options will vest on October 31, 2014 and the remaining shares subject to the option shall vest in thirty-six monthly installments thereafter. |
The Company has outstanding stock options under the 2011 Equity Incentive Plan. Outstanding employee stock options are subject to the provisions of the 2011 Equity Incentive Plan and the applicable option award agreement. Employee stock options vest over periods ranging between two and four years, and have a maximum term of ten years.
Options to Purchase Securities
The following table sets out, as at the date of this prospectus, information regarding outstanding options to purchase shares of our common stock which have been granted to our directors, executive officers, employees, consultants and past directors, executive officers, employees and consultants.
| Relationship to the Company | Number of Options(1) |
Securities Under Option |
Grant Date |
Expiry Date(2) |
Weighted Average Exercise Price(3) |
|||||||||
| All executive officers and past executive officers (3 individuals in total) |
1,702,622 | common stock |
April 9, 2014 to Nov. 20, 2014 |
April 9, 2024 to Nov. 20, 2024 |
$ | 0.50 | ||||||||
| All directors and past directors who are not also executive officers (2 individuals in total) |
500,000 | common stock |
— | Nov. 20, 2024 |
$ | 0.73 | ||||||||
| All other employees or past employees of Cohbar, Inc. (1 individual in total) |
36,438 | common stock |
April 9, 2014 |
April 9, 2024 |
$ | 0.26 | ||||||||
| All consultants and past consultants of Cohbar, Inc. (3 individuals in total) |
370,751 | common stock |
April 2, 2012 to Nov. 20, 2014 |
April 2, 2022 to Nov. 20, 2024 |
$ | 0.37 | ||||||||
| (1) | Represents the aggregate number of shares issuable upon exercise of all outstanding options held by the group. All options are granted under our 2011 Equity Incentive Plan. |
| (2) | All options granted under our 2011 Equity Incentive Plan expire ten years from the date of grant. |
| (3) | Represents the weighted average exercise price of all outstanding options held by the members of the group. Individual exercise prices per share range from $0.05 to $0.73. |
77
Severance and Change of Control Agreements
Other than those provisions contained in the executive employment agreements with Messrs. Stern and Biunno and Dr. Cundy, we do not have any severance or change in control agreements with any of our executive officers. The severance provisions for Messrs. Stern and Biunno and Dr. Cundy provide that each is entitled to a severance payment equal to fifty percent (50%) of his then current annual base salary in certain events of termination. Additionally, Mr. Stern and Dr. Cundy are each entitled to vesting acceleration of any options that would have become exercisable during the 12 months following such termination and reimbursement for any COBRA coverage elected for themselves and the members of their immediate families for a period of six months following termination. For a description of certain events that trigger immediate vesting of outstanding option awards see the sections captioned “Change in Control Provisions” in the summary of our 2011 Equity Incentive Plan above.
Compensation Committee Interlocks and Insider Participation
During the fiscal years ended December 31, 2012 and 2013, Jon Stern, Nir Barzilai and Pinchas Cohen, each of whom served as executive officers during such periods, participated in our board of directors’ deliberations on executive compensation. None of our executive officers serves as a member of the board of directors or compensation committee of any entity that has one or more executive officers serving on our board of directors or compensation committee.
78
CERTAIN RELATIONSHIPS AND RELATED PERSON TRANSACTIONS
In addition to the compensation arrangements, At-will Employment, Confidential Information, Invention Assignment and Arbitration Agreements, change in control arrangements and indemnification arrangements discussed above in the sections entitled “Management” and “Executive Compensation,” the registration rights described below under “Description of Capital Stock – Registration Rights” the following is a description of each transaction since January 1, 2011 and each currently proposed transaction in which:
| • | we have been or are to be a participant; |
| • | the amount involved exceeded or exceeds the lesser of (i) $120,000 or (ii) one percent of the average of our total assets as of the fiscal years ended December 31, 2012 and 2013; and |
| • | any of our directors, executive officers or holders of more than 5% of our capital stock, or any immediate family member of or person sharing the household with any of these individuals had or will have a direct or indirect material interest. |
Series A Financing and Related Transactions
On May 31, 2011, we entered into a Series A Preferred Stock Purchase Agreement with an individual accredited investor. The Agreement required the investor to purchase a total of 10,129,680 shares of our Series A Preferred Stock for an aggregate purchase price of $10 million. The investor purchased 1,012,968 shares of Series A Preferred Stock for USD $1 million at the initial closing on May 31, 2011. The Agreement required the investor to purchase additional shares of Series A Preferred Stock on specified future dates, contingent upon certain milestones related to securing patent licenses from our founders’ academic institutions, which milestones were achieved in November 2011.
Although the investor acknowledged the Company’s achievement of the relevant milestones, he failed to purchase shares of Series A preferred stock in late 2011 and early 2012 as required. Ultimately the investor purchased only 2,785,662 shares of Series A preferred stock for an aggregate purchase price of $2.75 million. As a result of the investor’s failure to complete the purchases required by the agreement all the outstanding shares of Series A Preferred Stock held by him were automatically converted to 2,785,662 shares of common stock.
On October 28, 2013, Dr. Barzilai and Dr. Cohen purchased all 2,785,662 shares of common stock held by the investor pursuant to a Stock Purchase and Sale Agreement among the three parties. The agreement provided for sale to each of Drs. Barzilai and Cohen of 1,392,831 of the subject shares for an aggregate price payable by each purchaser of $5,000. The purchase price is subject to upward adjustment in an amount not to exceed $1,295,000 per purchaser. The purchase price adjustment is payable only from cash proceeds, if any, of a disposition of shares of our capital stock held by Dr. Barzilai or Dr. Cohen, as applicable. The obligations of Drs. Barzilai and Cohen to pay the adjusted purchase price to the investor are unsecured, and the agreement does not create any obligation on the part of Dr. Barzilai or Dr. Cohen to dispose of shares of our capital stock at any time.
Consulting Agreements
Drs. Cohen and Barzilai each provide consulting services to our company pursuant to agreements that provide for annual compensation of $42,000. Each agreement provides for an annual service term. The service term under the agreement with Dr. Cohen expires in September 2015, and the service term under the agreement with Dr. Barzilai expires in November 2015.
Convertible Bridge Note Financing
In January 2014 we issued convertible notes, each in the original principal amount of $70,000 to three investors, including Messrs. Stern and Fitzgerald. The convertible notes did not bear interest and had a maturity date of one year. Together with the convertible note, each investor received a warrant to purchase 6,982 shares of
79
our common stock at an exercise price of $0.50 per share. Each note was automatically converted to shares of our Series B preferred stock upon the closing of our Series B preferred stock financing in April 2014 at a conversion rate of $0.50 per share. The warrants expire on the earlier to occur of (i) January 9, 2019 and (ii) a liquidation event as such term is defined in our Amended and Restated Certificate of Incorporation.
Series B Preferred Stock Financing
We issued to investors, including certain related parties, an aggregate of 5,400,000 shares of our Series B preferred stock pursuant to the terms of a Series B Preferred Stock Purchase Agreement, dated April 11, 2014, for an aggregate purchase price (including conversion of the convertible promissory notes described above) of $2,700,000, or $0.50 per share of Series B Preferred Stock.
Put Agreements
In connection with our Series B preferred stock financing, each purchaser of our Series B preferred stock entered into a Put Agreement (each a “Put Agreement” and collectively the “Put Agreements”) pursuant to which such investor will be required, at our election, to purchase from the Company securities of the same type as those sold to investors under this offering, at the same price as the securities sold under this offering, up to a total purchase amount per investor equal to the total purchase price paid by such investor for the Series B preferred stock purchased in our Series B preferred stock financing.
The issuance and sale of the units pursuant to the Put Agreements (the “Put Units”) will be completed pursuant to an exemption from registration under the Securities Act, including the exemption provided under Section 4(a)(2) thereof, and Regulation D promulgated thereunder. The issuance and sale of the Put Units is subject to, and will be effective concurrently with, the closing of this offering. The Put Units are being sold at the same price as the units being offered in this offering. The common stock and the warrants included in the Put Units have the same terms as the common stock and the warrants included in the units being offered hereunder, except that the Put Units, the common stock and warrants included in the Put Units, and the shares of common stock issuable upon exercise of such warrants will not be registered under the Securities Act.
In connection with this offering we have exercised our Put Rights to require the purchase of an aggregate of 2,700,000 Put Units, consisting of 2,700,000 shares of common stock, together with warrants to purchase 1,350,000 shares at a price of $1.00 per Put Unit. In accordance with the Put Agreements following delivery of the notice of exercise, each Series B investor deposited the applicable purchase price for the Put Units into an escrow account established for such purpose. Concurrently with the closing of this offering the proceeds of the escrow account will be released to us and the Put Units will be issued to the investors pursuant to the Put Agreements.
Each share of our Series B preferred stock will be automatically converted into one share of our common stock upon the completion of this offering; except that, pursuant to the terms of our Amended and Restated Certificate of Incorporation, the conversion rate applicable to the Series B preferred stock held by any Series B preferred stockholder who fails to complete the purchase and sale of Put Units as required by the applicable Put Agreement shall be adjusted downward so that such non-performing investor will be entitled upon such conversion to receive one-half of one share of common stock for each share of Series B preferred stock held by such non-performing investor. As the escrowed funds will be released to us upon the closing of this offering without further action by the holders of our Series B preferred stock, no additional actions of the holders of our Series B preferred stock are required to comply with their obligations under the Put Agreements. As such, there will be no downward adjustment of the common shares issuable upon conversion of the Series B preferred stock.
80
The following table summarizes the Series B preferred stock purchased by related parties pursuant to the Series B preferred stock purchase agreement described in this section and the purchase amount to which such investors have committed pursuant to the Put Agreements. The terms of these purchases were the same as those made available to unaffiliated purchasers.
| Related Person |
Shares of Series B Preferred Stock |
Put Commitment Amount |
||||||
| Albion J. Fitzgerald |
1,000,000 | (1) | $ | 500,000 | ||||
| Jon Stern |
300,000 | (1) | $ | 150,000 | ||||
| Hastings Private Investments Ltd. |
1,000,000 | $ | 500,000 | |||||
| (1) | Includes shares received upon conversion of convertible promissory note. |
Stockholder Agreements
In connection with sales of our preferred stock, we entered into agreements that grant customary preferred stock rights to our preferred stock investors, including our directors, executive officer and holders of more than 5% of our outstanding stock described above as holders of our Series B preferred stock. These rights include registration rights, rights of first refusal, co-sale rights with respect to certain stock transfers, a voting agreement providing for the election of investor designees to our board of directors, information rights and other similar rights. Our Investors’ Rights Agreement, which contains the registration rights and many of the other rights described above, is filed as an exhibit to the registration statement of which this prospectus is a part. All of these rights, other than the registration rights, will terminate effective upon the closing of this offering.
Upon the closing of this offering, assuming the sale and issuance pursuant to the exercise of our Put Rights of 2,700,000 units and the conversion of our outstanding Series B preferred stock into 5,400,000 shares of common stock, holders of approximately 21,015,343 shares of our common stock will be entitled to rights with respect to the registration of these shares under the Securities Act. For a more detailed description of these registration rights, see “Description of Capital Stock – Registration Rights.”
Indemnification Agreements
We have entered into a standard form of indemnification agreement with each of our directors and executive officers. Under this agreement, we are obligated to indemnify the indemnitee to the fullest extent permitted by applicable law for all reasonable expenses (including attorneys’ fees and all other disbursements or expenses of the types customarily incurred in connection with threatened, pending or completed proceedings), judgments, fines and amounts paid in settlement actually and reasonably incurred by the indemnitee or on the indemnitee’s behalf arising out of or connected with the indemnitee’s service as a director or officer or the indemnitee’s service in another capacity at our request or direction, provided that the Indemnitee acted in good faith and in a manner the indemnitee reasonably believed to be not opposed to our best interests and, with respect to any criminal action or proceeding, the indemnitee had no reasonable cause to believe that his or her conduct was unlawful. We are also obligated to advance all reasonable expenses (including attorneys’ fees and all other disbursements or expenses of the types customarily incurred in connection with threatened, pending or completed proceedings) incurred by the indemnitee in connection with threatened, pending or completed proceedings, and our standard form of indemnification agreement includes an undertaking by the indemnitee to repay any advance to the extent that it is ultimately determined that the indemnitee is not entitled to indemnification. If a claim for indemnification under our standard form of indemnification agreement is unavailable to the indemnitee, then we are obligated to contribute to the amounts incurred by the indemnitee, whether for expenses, judgments, fines or amounts paid or to be paid in settlement, an amount in proportion to the relative benefits received by us and the indemnitee from the events and transactions from which such action arose, and in connection with the events and transactions from which such action arose. The rights of the indemnitee under our standard form of indemnification agreement are in addition to any other rights that the indemnitee may have under our second
81
amended and restated certificate of incorporation and our amended and restated bylaws, in each case, that will be in effect upon completion of this offering, and any resolutions adopted pursuant thereto. We are not obligated to make any payment under our standard form of indemnification agreement to the extent payment is actually made to the indemnitee under any insurance policy or any other method outside of the agreement.
Policies and Procedures for Related Person Transactions
Our board of directors has adopted a policy stating that our executive officers, directors, nominees for election as a director, beneficial owners of more than 5% of any class of our common stock and any members of the immediate family of any of the foregoing persons are not permitted to enter into a related person transaction with us without the prior consent of the independent members of our board of directors. Under this policy, any request for us to enter into a transaction with an executive officer, director, nominee for election as a director, beneficial owner of more than 5% of any class of our common stock or any member of the immediate family of any of the foregoing persons, in which the amount involved exceeds $120,000 and pursuant to which such person would have a direct or indirect interest must first be presented to the independent members of our board of directors for review, consideration and approval. In approving or rejecting any such proposal, the independent members of our board of directors are to consider the material facts of the transaction, including, but not limited to, whether the transaction is on terms no less favorable than terms generally available to an unaffiliated third party under the same or similar circumstances and the extent of the related person’s interest in the transaction.
82
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth information regarding beneficial ownership of our common stock as of December 18, 2014, the most recent practicable date for computing beneficial ownership, by:
| • | each of our named executive officers; |
| • | each of our directors; |
| • | each person, or group of affiliated persons, known by us to beneficially own more than 5% of our common stock; and |
| • | all of our directors and executive officers as a group. |
We have determined beneficial ownership in accordance with the rules of the SEC. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting or investment power with respect to those securities. Unless otherwise indicated, the persons or entities identified in this table have sole voting and investment power with respect to all shares shown as beneficially owned by them, subject to applicable community property laws.
Applicable percentage ownership before the offering is based on 18,315,343 shares of our common stock issued and outstanding as of December 18, 2014, assuming the conversion of our outstanding preferred stock into 5,400,000 shares of common stock. In computing the number of shares of common stock beneficially owned by a person or entity and the percentage of our common stock owned by that person or entity, we deemed to be outstanding all shares of common stock subject to options, warrants or other convertible securities held by that person or entity that are currently exercisable or convertible, or will be exercisable or convertible within 60 days of December 18, 2014. We did not deem these shares outstanding, however, for the purpose of computing the percentage ownership of any other person. The percentage of shares beneficially owned after the offering is based on 32,265,343 shares of our common stock outstanding after the effectiveness of the offering, assuming sale of 11,250,000 units in the offering, and after giving effect to (i) the conversion of our outstanding preferred stock into 5,400,000 shares of common stock and (ii) sale and issuance of an aggregate of 2,700,000 shares of our common stock included in the units issued pursuant to the exercise of our Put Rights.
| Name and Address of Beneficial Owner |
Number of Shares Beneficially Owned |
Percentage of Shares Beneficially Owned |
||||||||||||||
| Before Offering |
After Offering |
Before Offering |
After Offering |
|||||||||||||
| Pinchas Cohen(1) |
5,444,703 | 5,444,703 | 29.73 | % | 16.87 | % | ||||||||||
| Nir Barzilai(1) |
5,039,516 | 5,039,516 | 27.52 | % | 15.62 | % | ||||||||||
| Jon Stern(1)(2) |
1,383,021 | 1,608,021 | 7.13 | % | 4.81 | % | ||||||||||
| Jeffrey Biunno(1)(3) |
74,013 | 113,864 | * | * | ||||||||||||
| Albion Fitzgerald(1)(4) |
1,022,607 | 1,772,607 | 5.58 | % | 5.45 | % | ||||||||||
| Marc Goldberg(1)(5) |
15,625 | 15,625 | * | * | ||||||||||||
| Hastings Private Investments Ltd.(6) |
1,000,000 | 1,750,000 | 5.46 | % | 5.38 | % | ||||||||||
| Directors and executive officers as a group (7 people) |
12,979,485 | 13,994,336 | 66.63 | % | 41.41 | % | ||||||||||
| (1) | The address for each director and executive officer is c/o Cohbar, Inc., 2265 E. Foothill Blvd., Pasadena, California 91107 |
| (2) | Shares beneficially owned before the offering includes: (i) 300,000 shares of Series B preferred stock subject to conversion to common stock on completion of the offering; (ii) 278,964 shares of common stock subject to stock options exercisable within 60 days of December 18, 2014; and (iii) 804,057 shares of common stock subject to currently exercisable warrants. Shares beneficially owned after the offering includes the above securities, together with 150,000 units to be sold and issued to Mr. Stern pursuant to the exercise of our Put Rights, consisting of 150,000 shares of our common stock and warrants to purchase 75,000 shares of our common stock. |
83
| (3) | Shares beneficially owned before the offering consists of 74,013 shares of common stock subject to stock options exercisable within 60 days of December 18, 2014. Shares beneficially owned after the offering includes an additional 39,851 shares of common stock subject to stock options that will satisfy a performance vesting component upon completion of the offering and that will become exercisable on the basis of a time vesting component within 60 days of December 18, 2014. |
| (4) | Shares beneficially owned before the offering includes: (i) 1,000,000 shares of Series B preferred stock subject to conversion to common stock on completion of the offering; (ii) 6,982 shares of common stock subject to a currently exercisable warrant; and (iii) 15,625 shares of common stock subject to stock options exercisable within 60 days of December 18, 2014. Shares beneficially owned after the offering includes the above securities, together with 500,000 units to be sold and issued to Mr. Fitzgerald pursuant to the exercise of our Put Rights, consisting of 500,000 shares of our common stock and warrants to purchase 250,000 shares of our common stock. |
| (5) | Shares beneficially owned before and after the offering consist of 15,625 shares of common stock subject to stock options exercisable within 60 days of December 18, 2014. |
| (6) | Shares beneficially owned before the offering includes 1,000,000 shares of Series B preferred stock subject to conversion to common stock on completion of the offering. Shares beneficially owned after the offering includes the above securities, together with 500,000 units to be sold and issued to Hastings Private Investments Ltd. pursuant to the exercise of our Put Rights, consisting of 500,000 shares of our common stock and warrants to purchase 250,000 shares of our common stock. The Securities of Hastings Private Investments Ltd. are beneficially owned by the Hinrich Foundation, which is founded and controlled by Mr. Merle Hinrich. The address for Hastings Private Investments Ltd. is 26/F Southmark Tower B, 11 Hip Ying Street Wong Chuk Hang, Hong Kong |
| * | less than 1.0% |
84
General
The following descriptions of our capital stock and certain provisions of our certificate of incorporation and bylaws are summaries and are qualified by reference to the Third Amended and Restated Certificate of Incorporation and the Amended and Restated Bylaws that we intend to adopt, and we expect our stockholders will approve, prior to the completion of the offering for effectiveness upon completion of this offering. Copies of these documents are filed as exhibits to our Registration Statement, of which this prospectus forms a part.
As of the date of this prospectus, we had an aggregate of 18,315,343 shares of our common stock outstanding assuming, as of such date, the conversion of each share of our Series B preferred stock into one share of common stock. Our outstanding capital stock was held by 28 stockholders of record as of the date of this prospectus.
Additionally, as of such date, there were:
| • | 2,609,811 shares of common stock issuable upon exercise of options granted under our 2011 Stock Plan, with a weighted average exercise price of $0.52 per share; |
| • | 20,946 shares issuable upon exercise of outstanding common stock purchase warrants at an exercise price of $0.50 per share; |
| • | 897,075 shares issuable upon exercise of the common stock purchase warrants issued at an exercise price of $0.26 per share; and |
| • | 15,596 shares issuable upon exercise of a common stock purchase warrant at an exercise price of $0.99 per share. |
See “Prospectus Summary – Exercise of Company Put Rights” and “– Series B Preferred Stock Conversion”.
We expect that our Third Amended and Restated Certificate of Incorporation will provide that, upon the completion of this offering, our authorized capital stock will consist of 80,000,000 shares, all with a par value of $0.001 per share, of which:
| • | 75,000,000 shares are designated as common stock; and |
| • | 5,000,000 shares are designated as preferred stock. |
Common Stock
Dividend Rights
Subject to any preferences that may be applicable to any then outstanding shares of preferred stock, holders of our common stock are entitled to receive dividends of cash, property or shares of our capital stock that we pay or distribute out of funds legally available if our board of directors, in its discretion, determines to issue dividends and only then at the times and in the amounts that our board of directors may determine. For further information on our dividend policy, see “Dividend Policy” above.
Voting Rights
Each holder of our common stock is entitled to one vote for each share of common stock held by such holder on all matters on which stockholders generally are entitled to vote, provided that holders of common stock are not entitled to vote on amendments to our second amended and restated certificate of incorporation related solely to the terms of one or more outstanding series of preferred stock if the holders of such series are entitled to vote thereon, unless required by law. Our stockholders do not have cumulative voting rights in the election of directors. Accordingly, subject to the preferences that may be applicable to any then outstanding shares of preferred stock, holders of a majority of the voting shares are able to elect all of the directors.
85
Liquidation
In the event of our dissolution or liquidation, whether voluntary or involuntary, holders of our common stock will be entitled to share ratably in the net assets legally available for distribution to stockholders after the payment of all of our debts and other liabilities and subject to any preferential or other rights of any then outstanding shares of preferred stock.
Rights and Preferences
Holders of our common stock have no preemptive, conversion, subscription or other rights, and there are no redemption or sinking fund provisions applicable to our common stock. The rights, preferences and privileges of the holders of our common stock are subject to and may be adversely affected by the rights of the holders of shares of any series of our preferred stock that we may designate in the future.
Preferred Stock
As of the date of this prospectus, we had outstanding 5,400,000 shares of Series B preferred stock. All shares of our Series B preferred stock will be automatically converted into shares of our common stock upon the completion of this offering.
Pursuant to our Third Amended and Restated Certificate of Incorporation to be effective upon completion of this offering, our board of directors will have the authority, without further action by our stockholders, to issue up to 5,000,000 shares of preferred stock in one or more series and to fix the rights, preferences, privileges and restrictions thereof. These rights, preferences and privileges could include dividend rights, conversion rights, voting rights, terms of redemption, liquidation preferences, sinking fund terms and the number of shares constituting any series or the designation of such series, any or all of which may be greater than the rights of common stock. The issuance of preferred stock by us could adversely affect the voting power of holders of common stock and the likelihood that such holders will receive dividend payments and payments upon liquidation. In addition, the issuance of preferred stock could have the effect of delaying, deferring or preventing a change of control of our company or other corporate action. Upon the closing of this offering, no shares of preferred stock will be outstanding, and we have no present plan to issue any shares of preferred stock.
Warrants
Outstanding Warrants
As of the date of this prospectus, we had outstanding warrants to purchase an aggregate of 933,617 shares of our common stock as follows:
| Number of Shares Subject to Warrant (#) |
Exercise Price Per Share ($) |
Current Expiration Date |
||||||
| 20,946 |
$ | 0.50 | 1/09/2019 | |||||
| 797,075 |
$ | 0.26 | 7/24/2014 | (1) | ||||
| 15,596 |
$ | 0.99 | 8/24/2014 | |||||
| 100,000 |
$ | 0.26 | 7/24/2019 | (1) | ||||
| (1) | Warrants expire on the earliest to occur of (i) the indicated expiration date or (ii) a liquidation event, as defined in our Certificate of Incorporation. A liquidation event includes (a) any voluntary or involuntary liquidation, dissolution or winding up of our company, (b) a merger, consolidation or reorganization involving our company in which the holders of our capital stock immediately prior to such transaction hold less than a majority of the shares of capital stock of the surviving corporation immediately following such transaction or (c) the sale, lease, transfer, exclusive license or other disposition of substantially all of our assets. |
86
The table above does not include (i) warrants to purchase up to 1,350,000 shares of common stock issuable upon exercise of warrants included with the units to be issued pursuant to the exercise of our Put Rights in connection with this offering, (ii) warrants to purchase 5,625,000 shares of common stock anticipated to be issued in this offering as part of the units, (iii) up to 787,500 shares of common stock and warrants to purchase up to 393,750 shares of common stock issuable upon exercise of the unit options to be issued to our agents upon closing of the offering as compensation. The agent’s unit options will have an exercise price of $1.00 per unit and shall be exercisable for a period of 18 months following the closing. The warrants included in the units issued pursuant to the exercise of our Put Rights will have an exercise price of $2.00 per share and shall be exercisable for a period of 24 months following the closing, subject to our right to accelerate the expiration date of the warrants on the terms described below.
Warrants to be Issued in this Offering
Each unit issued in this offering includes one half of one common stock purchase warrant. The warrants issued in this offering will be governed by the terms of a warrant indenture that we will enter into with CST Trust Company, as the warrant agent, on or prior to the date of the issuance of the warrants. Each whole warrant will entitle its purchaser to purchase one share of our common stock at a price equal to $2.00 per share at any time for up to 24 months after the closing of this offering. The exercise price of the warrants was determined by negotiation between us and the agent. If the volume weighted average trading price of our common stock on the TSX-V is equal to or exceeds $3.00 per share for 20 consecutive trading days after the date on which our common stock is first traded on the TSX-V, we will have the right and option, exercisable at our sole discretion, to accelerate the expiration time of the warrants by providing written notice to each registered holder of warrants within five (5) business days and issuing a press release to the effect that the warrants will expire at 5:00 p.m. (Toronto time) on the date specified in such notice and press release, provided that such date shall not be less than 30 days following the date of such notice and press release. The holder of a warrant will not be deemed a holder of our underlying common stock until the warrant is exercised.
Warrant holders resident in the United States may exercise warrants only if the issuance of the common shares upon exercise of the warrants is covered by an effective registration statement, or an exemption from registration is available under the Securities Act and the securities laws of the state in which the holder resides. We intend to use commercially reasonable efforts to have the registration statement, of which this prospectus forms a part, effective when the warrants are exercised.
Investors should be aware; however, that we cannot provide any assurance that state exemptions will be available to us or that we will have an effective registration statement in place. Under no circumstances will we be required to pay any holder the net cash exercise value of any warrant regardless of whether an effective registration statement or an exemption from registration is available or not.
To exercise a warrant, a warrant holder must deliver the warrant certificate to the warrant agent on or before the warrant expiration date with the form on the reverse side of the warrant certificate fully executed and completed as instructed on the certificate, accompanied by payment of the full exercise price for the number of warrants being exercised. We will not issue any fractional shares of common stock upon exercise of the warrants. If we are not able to maintain the effectiveness of the registration statement covering the warrants and no exemption from registration is available, the holders of the warrants will not be able to exercise or resell their warrants or the underlying shares in the United States and they will expire unexercised.
All of the warrants described above contain provisions for the adjustment of their exercise price and the number of shares issuable upon exercise in the event of a stock dividend, reclassification, stock split, consolidation or similar event.
87
Stock Options
As of September 30, 2014 we had options outstanding under our 2011 Stock Plan to purchase an aggregate of 1,225,219 shares of our common stock, with a weighted-exercise average exercise price of $0.23 per share. As of the date of this prospectus, we had options outstanding under our 2011 Stock Plan to purchase an aggregate of 2,609,811 shares of our common stock, with a weighted-exercise price of $0.52 per share.
Registration Rights
After the closing of this offering, assuming the sale and issuance pursuant to the exercise of our Put Rights of 2,700,000 units and the conversion of our outstanding Series B preferred stock into 5,400,000 shares of common stock, the holders of approximately 21,015,343 shares of our common stock (Registrable Securities) will be entitled to rights with respect to the registration of these shares under the Securities Act, as described below. These registration rights are contained in our Investors’ Rights Agreement (IRA), dated as of April 11, 2014.
Mandatory Registration
We have agreed pursuant to the IRA to prepare and file a registration statement covering the resale of the Registrable Securities on or prior to the date the market standoff agreement included in our Investor Rights Agreement will expire (which we anticipate will be the 180th day after the filing of final prospectus related to the offering) and to use our best efforts to cause such registration statement to be declared effective as promptly as possible thereafter and to maintain such registration statement continuously effective until all Registrable Securities have been sold, or may be sold under Rule 144 without volume or manner of sale restrictions, and without requirement for us to maintain availability of current public information.
Expenses of Registration Rights
We will pay the expenses incurred by the holders in connection with the registration described above.
Expiration of Registration Rights
Registration rights under our IRA generally will expire on the earlier of (i) five years following the closing of the offering, or, (ii) with respect to any particular stockholder, when such stockholder is able to sell all of his or her Registrable Securities pursuant to Rule 144 of the Securities Act during any 90-day period.
Delaware Anti-Takeover Law and Provisions of our Second Amended and Restated Certificate of Incorporation and Second Amended and Restated Bylaws
Delaware Anti-Takeover Law
As a result of this offering, we may become subject to Section 203 of the Delaware General Corporation Law, which generally prohibits a Delaware corporation that has a class of voting stock that is listed on a “national securities exchange” or is held of record by more than 2,000 stockholders from engaging in a “business combination” with an “interested stockholder” for a period of three years following the date that such stockholder became an interested stockholder, unless:
| • | before such date, the board of directors of the corporation approved either the business combination or the transaction that resulted in the stockholder becoming an interested stockholder; |
| • | upon completion of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction began, excluding for purposes of determining the voting stock outstanding (but not the outstanding voting stock owned by the interested stockholder) those shares owned (i) by persons who are directors and also officers and (ii) employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or |
88
| • | on or after such date, the business combination is approved by the board of directors and authorized at an annual or special meeting of the stockholders, and not by written consent, by the affirmative vote of at least 66 2⁄3% of the outstanding voting stock that is not owned by the interested stockholder. |
For purposes of Section 203, “business combination” includes:
| • | any merger or consolidation involving the corporation and the interested stockholder; |
| • | any sale, transfer, pledge or other disposition of 10% or more of the assets of the corporation involving the interested stockholder; |
| • | subject to exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; or |
| • | the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation. |
In general, Section 203 defines an “interested stockholder” as an entity or person who, together with the person’s affiliates and associates, beneficially owns, or within three years prior to the time of determination of interested stockholder status did own, 15% or more of the outstanding voting stock of the corporation.
The TSX-V does not constitute a “national securities exchange” for purposes of Section 203. However, in the event that as a result of the offering our common stock is held of record by more than 2,000 stockholders or if the common stock is listed on an exchange that constitutes a national securities exchange within the meaning of Section 203, we would become subject to the foregoing restrictions.
Third Amended and Restated Certificate of Incorporation and Amended and Restated Bylaws
Prior to the completion of this offering we expect to adopt, and our stockholders are expected to approve for effectiveness upon completion of the offering, a third amended and restated certificate of incorporation and amended and restated bylaws. The provisions of our certificate of incorporation and amended and restated bylaws must conform to the requirements of applicable listing requirements and corporate governance rules of the TSX-V. Subject to such compliance, we may determine to adopt provisions which may delay, defer, prevent or render more difficult a takeover attempt that our stockholders might consider in their best interests. Even in the absence of a takeover attempt, the existence of these provisions may adversely affect the market value of our common stock if they are viewed as discouraging takeover attempts in the future. These may include:
| • | that the authorized number of directors may be changed only by resolution of the board of directors; |
| • | that all vacancies, including newly created directorships, may, except as otherwise required by law, be filled by the affirmative vote of a majority of directors then in office, even if such number is less than a quorum; |
| • | a requirement that any action to be taken by our stockholders be effected at a duly called annual or special meeting of stockholders and not by written consent; |
| • | that stockholders seeking to present proposals before a meeting of stockholders or to nominate candidates for election as directors at a meeting of stockholders must provide notice in writing in a timely manner, and also specify requirements as to the form and content of a stockholder’s notice; |
| • | the absence of cumulative voting rights, therefore allowing the holders of a majority of the shares of our common stock entitled to vote in any election of directors to elect all of the directors standing for election, if they should so choose; |
| • | limitations on the ability of our stockholders to require that a special meeting of stockholders be held; |
| • | limitations on the liability of, and provision of indemnification to, our directors and officers; |
89
| • | authorization of our board of directors to issue shares of preferred stock and to determine the price and other terms of such shares, including preferences and voting rights applicable to such shares, without stockholder approval; and |
| • | the ability of our board to postpone or reschedule any previously scheduled special meeting of stockholders. |
Should we determine to adopt all or any of these provisions, it may be difficult for our existing stockholders to replace our board of directors or for another party to obtain control of us by replacing our board of directors. Since our board of directors has the power to retain and discharge our officers, these provisions could also make it more difficult for existing stockholders or another party to effect a change in management. In addition, the authorization of undesignated preferred stock makes it possible for our board of directors to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to change our control.
These provisions may have the effect of deterring unsolicited takeover attempts or delaying or preventing changes in control of our company or changes in management. They are intended to enhance our long-term value to our stockholders by increasing the likelihood of continued stability in the composition of our board of directors and its policies and discouraging certain types of transactions that may involve an actual or threatened acquisition of us. These provisions are also designed to reduce our vulnerability to an unsolicited acquisition proposal and to discourage certain tactics that may be used in proxy fights. However, such provisions could have the effect of discouraging others from making tender offers for our shares and, as a consequence, they also may inhibit fluctuations in the market price of our stock that could result from actual or rumored takeover attempts.
Transfer Agent and Registrar
The main transfer agent and registrar for our common stock is CST Trust Company in Vancouver, British Columbia, and the co-transfer agent and co-registrar for our common stock is American Stock Transfer & Trust Company, LLC in New York, New York. The agent and registrar for our warrants is CST Trust Company in Vancouver, British Columbia.
Stock Exchange Listing
The TSX-V has conditionally approved the listing of our common stock under the symbol “COB”. We do not currently intend to list our common stock on any exchange in the United States. The warrants will not be listed on any exchange.
90
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there has been no public market for our securities. Future sales of shares of our common stock in the public market, or the availability of shares of our common stock for sale, may adversely impact the market price of our common stock. In addition, because contractual and legal restrictions will result in only a limited number of shares being available for sale shortly after this offering, the sale of substantial amounts of common stock, including shares issued upon exercise of outstanding options and warrants, in the market after such restrictions lapse could adversely affect the market price for our common stock as well as our ability to raise capital through the sale of our equity securities.
Based on the number of shares of common stock outstanding as of the date this prospectus and assuming (i) the sale pursuant to the exercise of our Put Rights of units comprised of an aggregate of 2,700,000 shares of common stock and warrants to purchase 1,350,000 shares of common stock, (ii) sale of the minimum of 11,250,000 units in the offering, (iii) no exercise of other options or warrants, and (iv) conversion of each outstanding share of Series B preferred stock into one share of common stock, there will be 32,265,343 shares of our common stock outstanding upon completion of this offering. Of these outstanding shares, all of the shares sold in this offering (including all of the shares issuable upon exercise of the warrants sold in this offering) will be freely tradable without restriction or further registration under the Securities Act, except that the sale of any units purchased in this offering by our affiliates, as such term is defined in Rule 144 under the Securities Act, would be subject to the volume and manner of sale limitations of Rule 144, as described below.
The remaining 21,015,343 outstanding shares of our common stock will be deemed “restricted securities” as defined in Rule 144, as currently in effect, including the 12,434,219 shares of common stock held by our executive officers and directors immediately following completion of the offering.
Rule 144
In general, under Rule 144 as currently in effect, our affiliates or persons selling shares on behalf of our affiliates are entitled to sell within any three-month period beginning 90 days after the date of this prospectus, a number of shares that does not exceed 1% of the number of shares of common stock then outstanding so long as they have held such shares for six months. Affiliates must comply with the applicable holding period requirements, which is six months if we have been a reporting company for at least 90 days, and one year if we have not. In addition, any sales by affiliates under Rule 144 are also limited by manner of sale provisions and notice requirements and the availability of current public information about us.
The volume limitation, manner of sale and notice provisions described above will not apply to sales by non-affiliates. Under Rule 144 as currently in effect, a person who is not deemed to have been an affiliate of ours at any time during the three months preceding a sale, and who has beneficially owned restricted securities within the meaning of Rule 144 for at least six months (including any period of consecutive ownership of preceding non-affiliated holders) would be entitled to sell those shares, subject only to the availability of current public information about us. A non-affiliated person who has beneficially owned restricted securities within the meaning of Rule 144 for at least one year would be entitled to sell those shares without regard to the provisions of Rule 144.
For purposes of Rule 144, a non-affiliate is any person or entity who is not our affiliate at the time of sale and has not been our affiliate during the preceding three months.
At the closing of this offering, 5,531,124 shares of our common stock (assuming conversion in full of all outstanding shares of preferred stock) will be eligible for immediate sale by non-affiliates in reliance on Rule 144, as currently in effect, provided, however that we expect 2,431,124 of such shares will be subject to a 24 month lock-up agreement and the TSX-V seed share resale restrictions and will be held in escrow. See “Escrowed Securities and Securities Subject to Contractual Restriction on Transfer”.
91
Rule 701
Rule 701 generally allows a stockholder who has purchased shares of our common stock pursuant to a written compensatory plan or contract and who is not deemed to have been an affiliate of our company during the immediately preceding 90 days to sell such shares in reliance upon Rule 144, but without being required to comply with the public information, holding period, volume limitation or notice provisions of Rule 144. Rule 701 also permits affiliates of our company to sell their Rule 701 shares under Rule 144 without complying with the holding period requirements of Rule 144. Rule 701 requires all holders of shares issued in reliance on Rule 701, however, to wait until 90 days after the date of this prospectus before selling such shares pursuant to Rule 701. The SEC has indicated that Rule 701 will apply to typical stock options granted by an issuer before it becomes subject to the reporting requirements of the Exchange Act, along with the shares acquired upon exercise of such options, including exercises after the date of this prospectus.
Regulation S, Rule 904
Rule 904 of Regulation S of the Securities Act provides that shares owned by any person, other than persons deemed to be affiliated with us by virtue of their significant beneficial ownership of our shares, may be sold without registration outside the United States, provided the sale is accomplished in an offshore transaction, no directed selling efforts are made and certain other conditions are satisfied, as specified in Rule 904. In general, this means that the shares, including restricted shares, and including shares of our common stock held by our directors and officers who do not own a significant percentage of the shares of common stock, may be sold on the TSX-V or otherwise outside the United States. In the case of a sale of shares by an officer or director who is our affiliate solely by virtue of holding such position, there would be an additional requirement that no selling commission, fee or other remuneration is paid in connection with such sale other than a usual and customary broker’s commission.
Notwithstanding the foregoing, shares sold without registration in reliance on Rule 904 will continue to be “restricted securities” and may be resold to a purchaser in the United States only under an effective registration statement or pursuant to an applicable exemption from registration, such as the exemption provided under Rule 144, if available.
Stock Options
As of September 30, 2014, we had outstanding options to purchase an aggregate of 1,225,219 shares of our common stock, with a weighted-exercise average exercise price of $0.23 per share. As of the date of this prospectus, we had outstanding options to purchase an aggregate of 2,609,811 shares of our common stock, with a weighted-exercise price of $0.52 per share.
As soon as practicable after the closing of this offering, we intend to file one or more registration statements on Form S-8 under the Securities Act covering all of the shares of our common stock subject to outstanding options and the shares of our common stock reserved for issuance under our stock plans. We expect to file this registration statement as soon as permitted under the Securities Act. However, the shares registered on Form S-8 may be subject to the volume limitations and the manner of sale, notice and public information requirements of Rule 144, as well as the TSX-V escrow arrangements and seed share resale restrictions described below, and will not be eligible for resale until expiration of the lock-up and market standoff agreements to which they are subject.
Registration Rights
We have agreed to file a registration statement covering the resale of approximately 21,015,313 shares of our common stock held by certain stockholders or their transferees. We are obligated to file such registration statement on or prior to the date that is approximately 180 days following the date of this prospectus, and to use our best efforts to cause such registration statement to be declared effective as promptly as practicable thereafter. For a description of these registration rights, please see “Description of Capital Stock – Registration Rights.” If these shares are registered, they will be freely tradable without restriction under the Securities Act.
92
Lock-up agreements
Each of our directors, officers, and stockholders holding more than 5% of our outstanding capital stock immediately prior to the completion of the offering have agreed not to offer, sell, contract to sell, or otherwise dispose of our common stock or any securities convertible into or exchangeable for shares of our common stock, subject to specified exceptions, for a period of 180 days following completion of the offering. Additionally, stockholders holding an aggregate of 12,915,343 shares, including Drs. Barzilai and Cohen, have agreed not to offer, sell, contract to sell, or otherwise dispose of our common stock or any securities convertible into or exchangeable for shares of our common stock, subject to specified exceptions, for a period of 24 months following completion of the offering.
Escrowed Securities and Securities Subject to Contractual Restriction on Transfer
The following three sections describe restrictions on resale arising under the rules and regulations of applicable Canadian securities regulators and the TSX-V.
Principal’s Escrow
In accordance with National Policy 46-201 Escrow for Initial Public Offerings (National Policy 46-201), our Principals (as defined below) are required to deposit into escrow our equity securities and any securities that are convertible into our equity securities that they own or control (which we refer to as the “Principal’s Escrow”). “Principals” include all persons or companies that will, on the completion of this offering, fall into at least one of the following categories: (i) a person or company who acted as our promoter within two years before the date of this prospectus; (ii) our directors and/or senior officers; (iii) those who own and/or control more than 10% of our voting securities immediately before and after the completion of this offering if they also have appointed or have the right to appoint one or more of our directors or senior officers; (iv) those who own and/or control more than 20% of our voting securities immediately before and after the completion of this offering; (v) a company, trust, partnership or other entity more than 50% held by one or more Principals; and (vi) a Principal’s spouse and their relatives that live at the same address as the Principal.
A Principal that holds securities carrying less than 1% of the voting rights attached to our outstanding securities immediately after the completion of this offering will not be subject to the Principal’s Escrow.
Pursuant to the Principal’s Escrow, the Principals will deposit into escrow with CST Trust Company their shares of common stock, warrants and options to purchase shares of our common stock (which we refer to as the “Escrowed Securities”) which will be subject to escrow.
Upon completion of this offering we expect to be classified as an “emerging issuer” pursuant to National Policy 46-201 as our common stock is anticipated to be listed on Tier 2 of the TSX-V. In that event, 10% of the Escrowed Securities will be released from escrow upon receipt of notice from the TSX-V confirming the listing of our common stock on the TSX-V. The remaining 90% of the Escrowed Securities will be released from escrow in 15% tranches at six-month intervals over a 36-month period following receipt of such notice.
TSX-V Seed Share Resale Restrictions
Securities that were issued to parties other than our Principals prior to the completion of this offering will be subject to resale restrictions imposed by the TSX-V (which we refer to as the “TSX-V Seed Share Resale Restrictions”). The purchase price of such securities and the time of their purchase relative to the date of a receipt for this prospectus by the applicable Canadian securities regulators determines which TSX-V hold period applies. We expect a four month hold period to apply to the holders subject to the Seed Share Resale Restrictions. The TSX-V hold period does not apply to persons who are subject to the Principal’s Escrow as discussed above.
93
Summary of Escrow and Contractual Restrictions on Transfer
As of the date hereof, the following table sets out the number and percentage of our securities which will be subject to the Principal’s Escrow and TSX-V Seed Share Resale Restrictions upon the closing of this offering.
|
Number of Securities |
Percentage of Class Outstanding |
|||||||||||
| Designation of Class |
Prior to the Offering |
After the Offering |
||||||||||
| Common Stock |
14,865,343 | (1) | 78.38 | %(4) | 46.07 | %(6) | ||||||
| Options |
2,536,935 | (2) | 96.98 | % | 96.98 | % | ||||||
| Warrants |
1,236,039 | (3) | 98.21 | %(5) | 15.62 | %(7) | ||||||
| (1) | 12,434,219 shares of our common stock will be held in escrow under the Principal’s Escrow upon completion of the offering, assuming the conversion of each share of our Series B preferred stock held by our Principals into one share of common stock, and the sale and issuance to our Principals of an aggregate of 650,000 shares of common stock included in the units to be issued pursuant to the exercise of our Put Rights. 2,431,124 shares of our common stock will be subject to the TSX-V Seed Share Resale Restrictions and will be held in escrow. |
| (2) | 1,952,622 stock options will be held in escrow under the Principal’s Escrow. 584,313 stock options will be subject to the TSX-V Seed Share Resale Restrictions. |
| (3) | 1,136,039 common stock purchase warrants will be held in escrow under the Principal’s Escrow. 100,000 common stock purchase warrants will be subject to the TSX-V Seed Share Resale Restrictions. |
| (4) | This percentage is based on 18,965,343 shares of common stock issued and outstanding immediately prior to the effectiveness of the offering, assuming the conversion of each share of our Series B preferred stock held by our Principals into one share of common stock, and the sale and issuance of an aggregate of 650,000 shares of common stock included in the units to be issued to our Principals (but no other shareholders) pursuant to the exercise of our Put Rights. |
| (5) | This percentage is based on 1,258,617 common stock purchase warrants outstanding immediately prior to the effectiveness of the offering, assuming the sale and issuance of 325,000 common stock purchase warrants included in the units to be issued to our Principals (but no other shareholders) pursuant to the exercise of our Put Rights. |
| (6) | This percentage is based on 32,265,343 shares of our common stock issued and outstanding as of the effectiveness of the offering after giving effect to the conversion of all outstanding shares of our Series B preferred stock and the sale and issuance pursuant to the exercise of our Put Rights of an aggregate of 2,700,000 units comprised of an aggregate of 2,700,000 shares of common stock, together with warrants to purchase up to 1,350,000 shares of common stock. |
| (7) | This percentage is based on 7,908,617 warrants outstanding after the effectiveness of the offering, consisting of (i) 933,617 outstanding common stock purchase warrants, (ii) 1,350,000 common stock purchase warrants issuable pursuant to the exercise of our Put Rights and (iii) 5,625,000 common stock purchase warrants included in the units to be issued in this offering. |
94
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES
FOR NON-U.S. HOLDERS OF COMMON STOCK
This section is a summary of the material United States federal income tax consequences relating to the ownership and disposition of the shares of common stock and warrants by a non-U.S. holder (as defined below). This discussion only addresses shares of our common stock and warrants held as capital assets (generally, property held for investment) by non-U.S. holders who purchase such common stock and warrants in this offering. This summary does not purport to be a complete analysis of all potential tax consequences. The information provided below is based on provisions of the Internal Revenue Code, as amended (Code), and Treasury regulations promulgated thereunder, administrative rulings and judicial decisions, all as of the date hereof. These authorities may change, possibly on a retroactive basis, or the Internal Revenue Service (IRS), might interpret the existing authorities differently. Consequently, the U.S. tax consequences of owning or disposing of the shares of common stock and warrants could differ from those described below. For purposes of this summary, a “non-U.S. holder” is any beneficial holder of our common stock and warrants (other than an entity treated as a partnership for United States federal income tax purposes) that is not:
| • | an individual citizen or resident of the United States; |
| • | a corporation or other entity taxable as a corporation for United States federal income tax purposes, created or organized under the laws of the United States, any state thereof, or the District of Columbia; |
| • | a trust that (1) is subject to the primary supervision of a United States court and one or more United States persons have authority to control all substantial decisions of the trust or (2) has a valid election in effect under applicable Treasury regulations to be treated at a United States person; or |
| • | an estate whose income is subject to United States income tax regardless of source. |
If you are a non-U.S. citizen that is an individual, you may be a resident alien, as opposed to a nonresident alien, by virtue of being present in the United State for at least 31 days in the calendar year and for an aggregate of at least 183 days during a three-year period ending in the current calendar year. For these purposes, all the days present in the current year, one-third of the days present in the immediately preceding year, and one-sixth of the days present in the second preceding year are counted. Resident aliens are subject to United States federal income tax consequences of the sale, exchange or other disposition of the shares of common stock and warrants. If a partnership or other pass-through entity is a beneficial owner of the shares of common stock and warrants, the tax treatment of a partner in the partnership or an owner of the entity will depend upon the status of the partner or other owner and the activities of the partnership or other entity. Any partner in a partnership or member in a pass-through entity holding shares of our shares of common stock and warrants should consult its own tax advisor.
This summary generally does not address tax consequences that may be relevant to particular investors because of their specific circumstances, or because they are subject to special rules, including, without limitation, if the investor is a United States expatriate, a “controlled foreign corporation,” a “passive foreign investment company,” a corporation that accumulates earnings to avoid United States federal income tax, a dealer in securities or currencies, a financial institution, a regulated investment company, a real estate investment trust, a tax-exempt entity, an insurance company, a cooperative, a holder that owns or acquires 5% or more of our common stock, a person holding our common stock as part of a hedging, integrated, conversion or constructive sale transaction or straddle, a trader in securities that elects to use a mark-to-market method of accounting, a person liable for the alternative minimum tax, a person whose functional currency is other than the U.S. dollar, a person who acquired our common stock as compensation for services, and a partner or beneficial owner in a pass-through entity. Finally, this summary does not describe the effects of any applicable non-U.S., state or local laws, or except to the extent discussed below, the effects of any applicable gift or estate tax laws.
INVESTORS CONSIDERING THE PURCHASE OF THE SHARES OF COMMON STOCK AND WARRANTS SHOULD CONSULT THEIR OWN TAX ADVISORS REGARDING THE APPLICATION OF THE UNITED STATES FEDERAL INCOME AND ESTATE TAX LAWS TO THEIR PARTICULAR SITUATIONS AND THE CONSEQUENCES OF NON-U.S., STATE OR LOCAL LAWS, AND TAX TREATIES.
95
Allocation of Purchase Price
In acquiring the units, the purchasers will be acquiring ownership of the shares of common stock and the warrants represented by the units. The shares of common stock and warrants represented by the units are separate securities and, accordingly, the purchasers will be required to allocate the purchase price paid for the units between the shares of common stock and the warrants on a reasonable basis in order to determine their respective costs for purposes of federal income tax. We intend to allocate 89% of the public offering price of each unit as consideration for the issue of each share of common stock and 11% for the issue of each one-half warrant. Although we believe this allocation is reasonable, this allocation will not be binding on the Internal Revenue Service or any other tax authority and neither we nor our counsel express any opinion as to this allocation.
Dividends
We do not expect to declare or pay any dividends on our common stock in the foreseeable future. If we do pay dividends on shares of our common stock, however, such distributions will constitute dividends for United States federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under United States federal income tax principles. Distributions in excess of our current and accumulated earnings and profits will constitute a return of capital that is applied against and reduces, but not below zero, a non-U.S. holder’s adjusted tax basis in shares of our common stock. Any remaining excess will be treated as gain realized on the sale or other disposition of our common stock. See “– Sale of Common Stock.”
Any dividend paid to a non-U.S. holder on our common stock will generally be subject to United States withholding tax at a 30% rate. The withholding tax might not apply, however, or might apply at a reduced rate, under the terms of an applicable income tax treaty between the United States and the non-U.S. holder’s country of residence. You should consult your own tax advisors regarding your entitlement to benefits under a relevant income tax treaty. Generally, in order for us or our paying agent to withhold tax at a lower treaty rate, a non-U.S. holder must certify its entitlement to treaty benefits. A non-U.S. holder generally can meet this certification requirement by providing a Form W-8BEN (or any successor form) or appropriate substitute form to us or our transfer agent. If the non-U.S. holder holds our common stock through a financial institution or other agent acting on the holder’s behalf, the holder will be required to provide appropriate documentation to such agent. The holder’s agent will then be required to provide certification to us or our transfer agent, either directly or through other intermediaries. For payments made to a foreign partnership or other pass-through entity, the certification requirements generally apply to the partners or other owners rather than to the partnership or other entity, and the partnership or other entity must provide the partners’ or other owners’ documentation to us or our transfer agent. In the case of common stock held by a non-U.S. intermediary, the intermediary generally must provide an IRS Form W-8IMY (or any successor form) and satisfy the relevant certificate requirements of applicable Treasury regulations.
Dividends received by a non-U.S. holder that are effectively connected with a U.S. trade or business conducted by the non-U.S. holder, or, if an income tax treaty between the United States and the non-U.S. holder’s country of residence applies, are attributable to a permanent establishment maintained by the non-U.S. holder in the United States, are not subject to such withholding tax. To obtain this exemption, a non-U.S. holder must provide us with an IRS Form W-8ECI properly certifying such exemption. Such effectively connected dividends, although not subject to withholding tax, are generally taxed at the same graduated rates applicable to U.S. persons, net of certain deductions and credits. In addition to the graduated tax described above, dividends received by corporate non-U.S. holders that are effectively connected with a U.S. trade or business of the corporate non-U.S. holder may also be subject to a branch profits tax at a rate of 30% or such lower rate as may be specified by an applicable tax treaty.
If you are eligible for a reduced rate of United States federal withholding tax under an income tax treaty, you may obtain a refund or credit of any excess amounts withheld by filing an appropriate claim for a refund with the IRS in a timely manner.
96
Sale of Common Stock
Non-U.S. holders will generally not be subject to United States federal income tax on any gains realized on the sale, exchange or other disposition of common stock unless:
| • | the gain (1) is effectively connected with the conduct by the non-U.S. holder of a United States trade or business and (2) if in accordance with an applicable income tax treaty between the United States and the non-U.S. holder’s country of residence applies, the gain is attributable to a permanent establishment; |
| • | the non-U.S. holder is an individual who is present in the United States for 183 days or more in the taxable year of the sale, exchange or other disposition of our common stock, and certain other requirements are met (in which case the gain would be subject to a flat 30% tax, or such reduced rate as may be specified by an applicable income tax treaty, which may be offset by U.S. source capital losses, even though the individual is not considered a resident of the United States); or |
| • | the rules of the Foreign Investment in Real Property Tax Act, or FIRPTA, treat the gain as effectively connected with a United States trade or business. |
The FIRPTA rules will apply to a sale, exchange or other disposition of our common stock if we are, or were within the shorter of the five-year period preceding the disposition and the non-U.S. holder’s holding period a “U.S. real property holding corporation,” or USRPHC. In general, we would be a USRPHC if interests in United States real estate comprised at least half of our business assets. We do not believe that we are a USRPHC and we do not anticipate becoming one in the future. Even if we become a USRPHC, as long as our common stock is regularly traded on an established securities market, such common stock will be treated as United States real property interests only if a non-U.S. holder actually owns or constructively holds more than 5% of our outstanding common stock.
If any gain from the sale, exchange or other disposition of common stock, (1) is effectively connected with a United States trade or business conducted by a non-U.S. holder and (2) if an income tax treaty between the United States and the non-U.S. holder’s country of residence applies, is attributable to a permanent establishment (or, in the case of an individual, a fixed base) maintained by such non-U.S. holder in the United States, then the gain generally will be subject to United States federal income tax at the same graduated rates applicable to U.S. persons, net of certain deductions and credits. If the non-U.S. holder is a corporation, under certain circumstances, that portion of its earnings and profits that is effectively connected with its United States trade or business, subject to certain adjustments, generally would be subject to a “branch profits tax.” The branch profits tax rate is generally 30%, although an applicable income tax treaty between the United States and the non-U.S. holder’s country of residence might provide for a lower rate.
Tax Consequences to Non-U.S. Holders of the Exercise and Disposition of Warrants
Exercise of Warrants
A non-U.S. holder should not recognize gain or loss on exercise of a warrant. For U.S. federal income tax purposes, a non-U.S. holder’s initial tax basis in the share of common stock received on the exercise of a warrant should be equal to the sum of (a) such non-U.S. holder’s tax basis in such warrant plus (b) the exercise price paid by such U.S. holder on the exercise of such warrant. A non-U.S. holder’s holding period for the share of common stock received on the exercise of a warrant should begin on the date that such warrant is exercised by such non-U.S. holder.
In certain limited circumstances, a non-U.S. holder may be permitted to undertake a cashless exercise of warrants into common stock. The U.S. federal income tax treatment of a cashless exercise of warrants into common stock is unclear, and the tax consequences of a cashless exercise could differ from the consequences upon the exercise of a warrant described in the preceding paragraph. Non-U.S. holders should consult their own tax advisors regarding the U.S. federal income tax consequences of a cashless exercise of warrants.
97
Disposition of Warrants and Expiration of Warrants Without Exercise
Gain or loss realized by a non-U.S. holder as a result of a disposition of warrants, or loss realized as a result of a lapse or expiration of a warrant (which generally would be in an amount equal to such non-U.S. holder’s tax basis in the warrant) will be the same as the tax consequences relating to a disposition of shares of common stock. See “Sale of Common Stock.”
Certain Adjustments to the Warrants
Under Section 305 of the Code, an adjustment to the number of shares of common stock that will be issued on the exercise of the warrants, or an adjustment to the exercise price of the warrants, may be treated as a constructive distribution to a non-U.S. holder of the warrants if, and to the extent that, such adjustment has the effect of increasing such non-U.S. holder’s proportionate interest in the “earnings and profits” or assets of our Company, depending on the circumstances of such adjustment (for example, if such adjustment is to compensate for a distribution of cash or other property to stockholders of our Company). Adjustments to the exercise price of warrants made pursuant to a bona fide reasonable adjustment formula that has the effect of preventing dilution of the interest of the holders of the warrants should generally not be considered to result in a constructive distribution. Any such constructive distribution would be taxable whether or not there is an actual distribution of cash or other property. See “Dividends”.
United States Federal Estate Tax
The estates of nonresident alien individuals generally are subject to United States federal estate tax on property with a United States situs. Because we are a United States corporation, our common stock will be United States situs property and therefore will be included in the taxable estate of a nonresident alien decedent, unless an applicable tax treaty between the United States and the decedent’s country of residence provides otherwise.
Backup Withholding and Information Reporting
Generally, we must report annually to the IRS the amount of dividends paid to you, your name and address, and the amount of tax withheld, if any. A similar report will be sent to you. Pursuant to applicable income tax treaties or other agreements, the IRS may make these reports available to tax authorities in your country of residence.
Payments of dividends or of proceeds on the disposition of stock made to you may be subject to information reporting and backup withholding at a current rate of 28% unless you establish an exemption, for example, by properly certifying your non U.S. status on a Form W-8BEN, W-8BEN-E or another appropriate version of IRS Form W-8. Notwithstanding the foregoing, backup withholding and information reporting may apply if either we or our paying agent has actual knowledge, or reason to know, that you are a U.S. person.
Backup withholding is not an additional tax; rather, the U.S. income tax liability of persons subject to backup withholding will be reduced by the amount of tax withheld. If withholding results in an overpayment of taxes, a refund or credit may generally be obtained from the IRS, provided that the required information is furnished to the IRS in a timely manner.
Rules Affecting Taxation of our Common Stock Held by or through Foreign Entities
The Foreign Account Tax Compliance Act imposes withholding taxes on certain types of U.S. source income “withholdable payments” (including dividends, rents, gains from the sale of U.S. equity securities and certain interest payments) made to “foreign financial institutions” and certain other “non-financial foreign entities” unless various U.S. information reporting and due diligence requirements (generally relating to ownership by U.S. persons of interests in or accounts with those entities) have been satisfied. Withholding under
98
this legislation on withholdable payments to foreign financial institutions is required on payments made after June 30, 2014 and is expected to be required with respect to gross proceeds of a disposition of property that can produce U.S. source interest or dividends after December 31, 2016. Non-U.S. holders should consult their own tax advisors regarding the possible implications of this legislation on their investment.
Net Operating Losses
U.S. Income Tax laws impose special rules regarding the utilization of net operating losses in cases of some reorganizations and ownership changes. These rules can limit the amount of net operating losses that may be used to offset income after a corporation has undergone an ownership change. Investors should be aware that net operating losses that are, or may be, accumulated in the Company are subject to these rules. Limitations of net operating losses can lessen the value of the Company upon sale or other ownership changes.
EACH PROSPECTIVE INVESTOR SHOULD CONSULT ITS OWN TAX ADVISOR REGARDING THE PARTICULAR UNITED STATES FEDERAL, STATE, LOCAL AND FOREIGN TAX CONSEQUENCES OF PURCHASING, HOLDING AND DISPOSING OF THE SHARES OF COMMON STOCK AND WARRANTS, INCLUDING THE CONSEQUENCES OF ANY PROPOSED CHANGE IN APPLICABLE LAWS.
99
We have filed the Registration Statement of which this prospectus forms a part with the SEC solely for the purpose of registering the distribution of our securities in this offering outside the United States. The offering is an initial public offering in Canada subject to applicable Canadian securities laws and regulations.
This offering will not be conducted, and no sales of the units in this offering will be made, in the United States or any state, district, commonwealth or territory thereof, nor will offers or sales of the units in this offering be made to any person who is a “U.S. person” as defined under Rule 902(k) of Regulation S promulgated by the United States Securities and Exchange Commission under the Securities Act of 1933 or any other person in the United States. No commission or other form of compensation will be paid to any broker-dealer in the United States in connection with this offering.
We have engaged Haywood Securities Inc. as our agent in the offering. Haywood Securities Inc. is a registered and licensed dealer in Canada and is subject to Canadian dealer requirements in connection with this offering. The offering will be conducted on a “commercially reasonable efforts, minimum offering” basis pursuant to an agency agreement dated December 18, 2014, between us and the agent. The Agent must sell the number of units that will result in us achieving the minimum gross proceeds in the offering of $11,250,000, if any are sold. The Agent is required to use commercially reasonable efforts to sell the units offered. A “commercially reasonable efforts” offering does not obligate the agent to purchase any units from us as is the case in a “firm commitment” underwritten offering.
The offering will be marketed in all Provinces of Canada, other than Quebec. Subject to applicable law, Haywood Securities Inc. or its affiliates may also offer these units for sale in jurisdictions outside of Canada, except for the United States, provided such offer and sale will not require our company to comply with the registration, prospectus, filing, continuous, disclosure or similar requirements under the applicable securities laws of such other jurisdictions or pay any additional governmental filing fees which relate to such other jurisdictions.
Haywood Securities Inc. may appoint one or more investment dealers to form a selling group to participate in the placement of our units, provided that Haywood Securities Inc. shall at all times be the lead agent and sole bookrunner of the offering. At our request, Haywood Securities Inc. shall allocate units to other Canadian broker-dealers for placement by such broker-dealers. The commission paid to any selling group members will be paid by Haywood Securities Inc. from its commission.
Haywood Securities Inc. may terminate the agency agreement in its discretion if Haywood Securities Inc. is, among other things, not satisfied with its due diligence review and investigation of the Company, our financial position and assets or on the basis of its assessment of the state of the financial markets. Haywood Securities Inc. may also terminate the agency agreement in certain stated circumstances and upon the occurrence of certain stated events.
Subscriptions for the units will be subject to rejection or allotment in whole or in part and the right is reserved to close the subscription books at any time without notice. Subscription funds will be held in trust by the agent until closing of the offering. No funds shall be released to us until such time as the minimum gross proceeds of $11,250,000 are received. If the minimum proceeds of $11,250,000 are not received and we have not completed the offering on or before March 18, 2015, we will terminate the offering and the agent will promptly return all subscription funds to investors without interest or deduction.
The closing will occur as soon as practicable after the offering is fully subscribed, and the specific closing date will be established when a final receipt is issued by the principal regulator for the Canadian Prospectus pursuant to National Policy 11-202- Process for Prospectus Review in Multiple Jurisdictions. The closing date of
100
the offering is expected to be a date within 15 days of the issuance of a final receipt for the Canadian Prospectus. The date established for the closing is subject to postponement by agreement between us and the agent. The closing date may be postponed, among other circumstances, in connection with delays occasioned by clearing and settlement issues, prevailing market conditions, or investors withdrawing from the offering during the two-day right of withdrawal period stipulated under Canadian securities law, causing the offering not to be fully subscribed. We will publicly announce any postponement of the date established for the closing. We will terminate the offering if we have not received the minimum gross proceeds of $11,250,000 and completed the offering on or before March 18, 2015.
Except as may be otherwise agreed by us and the agent, it is expected that the shares of common stock and the common stock purchase warrants issued to purchasers as part of the offering will be issued in book-entry-only form in the name of CDS Clearing and Depository Services Inc. (CDS) or its nominee, CDS & Co. and will be deposited electronically on a non-certificated basis with CDS on the closing of the offering. Purchasers of common stock and common stock purchase warrants registered in the name of CDS or its nominee, will receive only a customer confirmation from the registered dealer who is a CDS participant and through whom the securities are purchased.
The warrants issued in this offering will be governed by the terms of a warrant indenture that we will enter into with CST Trust Company, as the warrant agent, on or prior to the date of the issuance of the warrants. Each whole warrant will entitle its purchaser to purchase one share of our common stock at a price equal to $2.00 per share at any time for up to 24 months after the closing of this offering, subject to our right to accelerate expiry of the warrants under certain circumstances. Warrants issued in the name of CDS, or its nominee, will be evidenced by a book-entry position on the register of warrantholders to be maintained by the Warrant Agent at its principal offices located in Vancouver, British Columbia.
As consideration for its services, Haywood Securities Inc. will receive: (i) a cash commission equal to 4% of the gross proceeds from the sale of units in the offering to certain specified purchasers and 7% of the gross proceeds from the sale of units in the offering to all other purchasers; (ii) unit options entitling the agent to purchase a number of units equal to 4% of the number of units sold under the offering to certain specified purchasers and 7% of the number of units sold under the offering to all other purchasers for a period of 18 months from the closing date at a price of $1.00 per unit; and (iii) a cash work fee of up to $30,000 payable in three equal monthly installments. No commission or other form of compensation will be paid to any broker-dealer in the United States in connection with this offering. Haywood Securities Inc. will also be reimbursed for its reasonable fees and expenses, including reasonable fees, disbursements and applicable taxes of legal counsel to Haywood Securities Inc. The expenses of the offering, (excluding agent’s fees and commissions and including agent’s expenses payable by us) will be approximately $250,000. We will pay all these expenses from the proceeds of the offering.
There is currently no market through which our securities may be sold, and purchasers may not be able to resell the securities purchased under this prospectus. See “Risk Factors” on page 11.
The TSX-V has conditionally approved the listing of our common stock under the symbol “COB”. Listing of our common stock will be subject to fulfilling all of the requirements of the TSX-V. We do not currently intend to list our common stock on any exchange in the United States. The warrants will not be listed on any exchange. This may affect the pricing of our securties in the secondary market, the transparency and availability of trading prices, the liquidity of the securities, and the extent of issuer regulation.
As of the date hereof, we are an “IPO Venture Issuer” (defined under National Instrument 41-101 as an issuer that does not have any of its securities listed or quoted, has not applied to list or quote any of its securities, and does not intend to apply to list or quote any of its securities, on the Toronto Stock Exchange, a U.S. marketplace, or a marketplace outside of Canada and the United States of America other than the Alternative Investment Market of the London Stock Exchange or the PLUS markets operated by PLUS Markets Group plc.).
101
Determination of Offering Price
Prior to the offering, there has been no public market for our securities. The initial public offering price of our units was negotiated between us and Haywood Securities Inc. In addition to prevailing market conditions, the factors considered in determining the initial public offering price were our financial information, our future prospects and the future prospects of our industry in general, our capital structure, estimates of our business potential and earnings prospects, the present state of our development and an assessment of our management and the consideration of the above factors in relation to market valuation of companies engaged in businesses and activities similar to ours.
An active trading market for our common stock may not develop. It is also possible that after the offering, the shares of common stock will not trade in the public market at or above the initial public offering price.
Indemnification
We have agreed to indemnify Haywood Securities Inc. against certain liabilities relating to the offering, including, without restriction, liabilities under the Securities Act and applicable Canadian provincial securities legislation, and liabilities arising from breaches of the representations and warranties contained in the agency agreement, and to contribute to payments that Haywood Securities Inc. may be required to make for these liabilities.
102
The legality of the securities being offered by this prospectus will be passed upon for us by Garvey Schubert Barer, Seattle, Washington. McCullough O’Connor Irwin LLP, Vancouver, British Columbia, will pass upon certain Canadian legal matters relating to the offering, and Thorsteinssons LLP, Vancouver, British Columbia, will pass upon certain Canadian federal tax matters relating to the offering. The agent is being represented in this offering by Dorsey & Whitney LLP, Toronto, Ontario, Canada (with respect to United States legal matters), and Wildeboer Dellelce LLP, Toronto, Ontario, Canada (with respect to Canadian legal matters).
Our financial statements as of December 31, 2013, 2012 and 2011, and for the years then ended, appearing in this prospectus and registration statement have been audited by Marcum LLP, an independent registered public accounting firm, as set forth in their report (which contains an explanatory paragraph relating to a substantial doubt about our ability to continue as a going concern as disclosed in Note 2 to the financial statements), appearing elsewhere herein and are included in reliance on such report given on the authority of said firm as experts in auditing and accounting.
As of the date hereof, the partners, counsel and associates of each of Garvey Schubert Barer, McCullough O’Connor Irwin LLP, Thorsteinssons LLP, Dorsey & Whitney LLP, and Wildeboer Dellelce LLP beneficially own directly or indirectly, respectively, less than 1% of our common stock or any common stock of any of our affiliates or associates.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a registration statement on Form S-1 (including exhibits and schedules thereto) under the Securities Act with respect to the shares of common stock being offered by this prospectus. This prospectus does not contain all of the information included in the registration statement. For further information about us and the securities described in this prospectus, we refer you to the registration statement. Statements contained in this prospectus as to the contents of any contract or any other document referred to are not necessarily complete, and in each instance, we refer you to the copy of the contract or other document filed as an exhibit to the registration statement. Each of these statements is qualified in all respects by this reference.
You may read our SEC filings, including the registration statement, over the Internet at the SEC’s website at www.sec.gov. You may also read and copy any document we file with the SEC at its public reference facilities at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. In addition, you may obtain copies of these documents at prescribed rates by writing to the Public Reference Section of the SEC at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the public reference facilities.
We intend to register a class of our common stock under Section 12 of the Exchange Act in connection with the offering. As a result, we will be subject to the information reporting requirements of the Securities Exchange Act and we will file reports, proxy statements and other information with the SEC. These reports, proxy statements and other information will be available for inspection and copying at the public reference facilities and website of the SEC referred to above. We also maintain a website at www.cohbar.com, at which you may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. The information contained on, connected to or that can be accessed through our website is not part of this prospectus. We have included our website address in this prospectus as an inactive textual reference only and not as an active hyperlink.
103
| Page | ||||
| Financial Statements for Fiscal Years Ended December 31, 2013, 2012 and 2011 |
||||
| F-2 | ||||
| F-3 | ||||
| F-4 | ||||
| F-5 | ||||
| F-6 | ||||
| F-7 | ||||
| Condensed Financial Statements for the Nine Months Ended September 30, 2014 and 2013 |
||||
| Balance Sheets as of September 30, 2014 (unaudited) and December 31, 2013 |
F-19 | |||
| Statements of Operations for the Three and Nine Months Ended September 30, 2014 and 2013 (unaudited) |
F-20 | |||
| Statements of Cash Flows for the Nine Months Ended September 30, 2014 and 2013 (unaudited) |
F-21 | |||
| F-22 | ||||
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Cohbar, Inc.
We have audited the accompanying balance sheets of Cohbar, Inc. (the “Company”) as of December 31, 2013, 2012 and 2011, and the related statements of operations, changes in stockholders’ equity (deficiency), and cash flows for the years then ended, and the related notes to the financial statements. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audits in accordance with standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free from material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Cohbar, Inc. as of December 31, 2013, 2012 and 2011, and the results of its operations and its cash flows for the years then ended in accordance with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has not generated any revenues and has incurred net losses since inception. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ Marcum LLP
Marcum LLP
New York, NY
August 6, 2014
F-2
Cohbar, Inc.
| December 31, 2013 |
December 31, 2012 |
December 31, 2011 |
||||||||||
| ASSETS | ||||||||||||
| Current assets: |
||||||||||||
| Cash |
$ | 145,170 | $ | 878,094 | $ | 518,863 | ||||||
| Restricted cash |
126,195 | — | — | |||||||||
| Prepaid expenses and other current assets |
15,124 | 14,970 | 1,600 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total current assets |
286,489 | 893,064 | 520,463 | |||||||||
| Property and equipment, net |
4,609 | 6,021 | 4,688 | |||||||||
| Deferred offering costs |
26,209 | — | — | |||||||||
| Other assets |
1,100 | 1,100 | 1,100 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total assets |
$ | 318,407 | $ | 900,185 | $ | 526,251 | ||||||
|
|
|
|
|
|
|
|||||||
| LIABILITIES AND STOCKHOLDERS’ (DEFICIENCY) EQUITY | ||||||||||||
| Current liabilities: |
||||||||||||
| Accounts payable |
$ | 54,645 | $ | 32,376 | $ | — | ||||||
| Accrued liabilities |
69,635 | 16,903 | — | |||||||||
| Accrued payroll and other compensation |
19,114 | 24,857 | 8,995 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total current liabilities |
143,394 | 74,136 | 8,995 | |||||||||
| Note payable, net of debt discount of $647 as of December 31, 2013 |
204,613 | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total liabilities |
348,007 | 74,136 | 8,995 | |||||||||
|
|
|
|
|
|
|
|||||||
| Commitments and contingencies |
||||||||||||
| Stockholders’ (deficiency) equity |
||||||||||||
| Preferred stock, $0.001 par value, Authorized- 8,000,000 shares (see Note 10); Issued and outstanding as of December 31, 2013, 2012 and 2011 as follows: |
||||||||||||
| Preferred stock – Series A – 0, 0 and 1,012,968 shares issued, and outstanding, respectively |
— | — | 1,013 | |||||||||
| Preferred stock – Series B – no shares issued, and outstanding, respectively |
— | — | — | |||||||||
| Common stock, $0.001 par value, Authorized – 37,000,000 shares (see Note 10); Issued and outstanding 12,915,343 shares as of December 31, 2013 and 2012 and 10,129,681 shares as of December 31, 2011 |
12,915 | 12,915 | 10,130 | |||||||||
| Additional paid-in capital |
2,594,128 | 2,577,136 | 799,026 | |||||||||
| Accumulated deficit |
(2,636,643 | ) | (1,764,002 | ) | (292,913 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total stockholders’ (deficiency) equity |
(29,600 | ) | 826,049 | 517,256 | ||||||||
|
|
|
|
|
|
|
|||||||
| Total liabilities and stockholders’ (deficiency) equity |
$ | 318,407 | $ | 900,185 | $ | 526,251 | ||||||
|
|
|
|
|
|
|
|||||||
The accompanying notes are an integral part of these financial statements.
F-3
Statements of Operations
| For The Years ended December 31, | ||||||||||||
| 2013 | 2012 | 2011 | ||||||||||
| Revenues |
$ | — | $ | — | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Operating expenses: |
||||||||||||
| Research and development |
478,256 | 854,292 | 109,301 | |||||||||
| General and administrative |
390,749 | 618,061 | 182,928 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
869,005 | 1,472,353 | 292,229 | |||||||||
|
|
|
|
|
|
|
|||||||
| Operating loss |
(869,005 | ) | (1,472,353 | ) | (292,229 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Other income (expense): |
||||||||||||
| Interest income |
505 | 1,264 | 488 | |||||||||
| Interest expense |
(4,003 | ) | — | — | ||||||||
| Amortization of debt discount |
(138 | ) | — | — | ||||||||
|
|
|
|
|
|
|
|||||||
| Total other income (expense) |
(3,636 | ) | 1,264 | 488 | ||||||||
|
|
|
|
|
|
|
|||||||
| Loss before income taxes |
(872,641 | ) | (1,471,089 | ) | (291,741 | ) | ||||||
| Income taxes |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
$ | (872,641 | ) | $ | (1,471,089 | ) | $ | (291,741 | ) | |||
|
|
|
|
|
|
|
|||||||
| Basic and diluted net loss per share |
$ | (0.07 | ) | $ | (0.12 | ) | $ | (0.03 | ) | |||
|
|
|
|
|
|
|
|||||||
| Weighted average common shares outstanding – basic and diluted |
12,915,343 | 12,094,629 | 10,129,681 | |||||||||
|
|
|
|
|
|
|
|||||||
The accompanying notes are an integral part of these financial statements.
F-4
Statements of Stockholders’ Equity (Deficiency)
| Preferred Stock | Stockholders’ Equity (Deficiency) | |||||||||||||||||||||||||||||||||||||||
| Preferred A | Preferred B | Common Stock | Total Stockholders’ Equity (Deficiency) |
|||||||||||||||||||||||||||||||||||||
| Number | Amount | Number | Amount | Total | Number | Amount | APIC | Accumulated Deficit |
||||||||||||||||||||||||||||||||
| Balance, December 31, 2010 |
— | $ | — | — | $ | — | $ | — | 10,129,681 | $ | 10,130 | $ | (5,683 | ) | $ | (1,172 | ) | $ | 3,275 | |||||||||||||||||||||
| Issuance of Preferred |
1,012,968 | 1,013 | — | — | 1,013 | — | — | 804,709 | — | 805,722 | ||||||||||||||||||||||||||||||
| Net Loss |
— | — | — | — | — | — | — | — | (291,741 | ) | (291,741 | ) | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Balance, December 31, 2011 |
1,012,968 | $ | 1,013 | — | $ | — | $ | 1,013 | 10,129,681 | $ | 10,130 | $ | 799,026 | $ | (292,913 | ) | $ | 517,256 | ||||||||||||||||||||||
| Stock Based Compensation |
— | — | — | — | — | — | — | 29,910 | — | 29,910 | ||||||||||||||||||||||||||||||
| Issuance of Preferred |
1,772,694 | 1,772 | — | — | 1,772 | — | — | 1,748,200 | — | 1,749,972 | ||||||||||||||||||||||||||||||
| Conversion of Preferred to Common Stock |
(2,785,662 | ) | (2,785 | ) | — | — | (2,785 | ) | 2,785,662 | 2,785 | — | — | — | |||||||||||||||||||||||||||
| Net Loss |
— | — | — | — | — | — | — | — | (1,471,089 | ) | (1,471,089 | ) | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Balance, December 31, 2012 |
— | $ | — | — | $ | — | $ | — | 12,915,343 | $ | 12,915 | $ | 2,577,136 | $ | (1,764,002 | ) | $ | 826,049 | ||||||||||||||||||||||
| Stock Based Compensation |
— | — | — | — | — | — | — | 16,207 | — | 16,207 | ||||||||||||||||||||||||||||||
| Debt Discounts – notes |
— | — | — | — | — | — | — | 785 | — | 785 | ||||||||||||||||||||||||||||||
| Net Loss |
— | — | — | — | — | — | — | — | (872,641 | ) | (872,641 | ) | ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Balance, December 31, 2013 |
— | $ | — | — | $ | — | $ | — | 12,915,343 | $ | 12,915 | $ | 2,594,128 | $ | (2,636,643 | ) | $ | (29,600 | ) | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
The accompanying notes are an integral part of these financial statements.
F-5
Statements of Cash Flows
| For The Years Ended December 31, | ||||||||||||
| 2013 | 2012 | 2011 | ||||||||||
| Cash flows from operating activities: |
||||||||||||
| Net loss |
$ | (872,641 | ) | $ | (1,471,089 | ) | $ | (291,741 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||
| Depreciation and amortization |
2,266 | 2,163 | 227 | |||||||||
| Loss on disposal of property and equipment |
334 | — | — | |||||||||
| Stock-based compensation |
16,207 | 29,910 | — | |||||||||
| Amortization of debt discount |
138 | — | — | |||||||||
| Changes in operating assets and liabilities: |
||||||||||||
| Restricted cash |
79,065 | — | — | |||||||||
| Prepaid expenses and other current assets |
(154 | ) | (13,369 | ) | (2,700 | ) | ||||||
| Accounts payable |
22,269 | 32,376 | — | |||||||||
| Accrued liabilities |
52,732 | 16,903 | — | |||||||||
| Accrued payroll and other compensation |
(5,743 | ) | 15,861 | 8,996 | ||||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in operating activities |
(705,527 | ) | (1,387,245 | ) | (285,218 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Cash flows from investing activities: |
||||||||||||
| Restricted cash |
(205,260 | ) | — | — | ||||||||
| Purchases of property and equipment |
(1,188 | ) | (3,496 | ) | (4,915 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in investing activities |
(206,448 | ) | (3,496 | ) | (4,915 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Cash flows from financing activities: |
||||||||||||
| Deferred offering costs |
(26,209 | ) | — | — | ||||||||
| Proceeds from the issuance of preferred stock, net |
— | 1,749,972 | 805,722 | |||||||||
| Proceeds from note payable |
205,260 | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by financing activities |
179,051 | 1,749,972 | 805,722 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net (decrease) increase in cash |
(732,924 | ) | 359,231 | 515,589 | ||||||||
| Cash at beginning of year |
878,094 | 518,863 | 3,274 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash at end of year |
$ | 145,170 | $ | 878,094 | $ | 518,863 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental disclosure of cash flow information: |
||||||||||||
| Cash paid: |
||||||||||||
| Income taxes |
$ | 2,441 | $ | — | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Non-cash investing and financing activities: |
||||||||||||
| Warrants issued in connection with note payable |
$ | 785 | $ | — | $ | — | ||||||
| Conversion of preferred stock to common stock |
$ | — | $ | 765 | $ | — | ||||||
The accompanying notes are an integral part of these financial statements.
F-6
COHBAR, INC.
FOR THE YEARS ENDED DECEMBER 31, 2013, 2012 AND 2011
NOTE 1 – BUSINESS ORGANIZATION AND NATURE OF OPERATIONS
Cohbar, Inc. (“Cohbar” or the “Company”) is a research stage biotechnology company focused on the discovery and development of novel peptide-based therapeutics for the treatment of diseases with significant unmet need. The Company’s research focuses specifically on the biology of Mitochondrial-Derived Peptides, or MDPs. To date, the Company has conducted investigational research into MDPs to evaluate their therapeutic potential and has identified four MDPs for possible advancement into drug candidate programs targeting one or more indications from a variety of diseases, including cancer, Alzheimer’s disease, atherosclerosis and certain metabolic disorders such as Type 2 diabetes and obesity.
The Company’s primary activities since inception have been the development and implementation of its business plans, negotiating inbound intellectual property licenses and other agreements, raising capital and conducting research on its MDPs. To date, the Company has not generated any revenues from operations.
Cohbar was founded in October 2007 as a limited liability company. In September 2009, the Company converted from a limited liability company into Cohbar, Inc., a Delaware corporation. To date, the Company has funded its business with the proceeds of private placements of equity and debt securities.
In April 2014, the Company effected a 3.6437695-for-1 stock split of its issued and outstanding shares of common stock. All references in these financial statements to the number of shares, options and other common stock equivalents, price per share and weighted-average number of shares outstanding of common stock have been adjusted to retroactively reflect the effect of the stock split.
NOTE 2 – GOING CONCERN AND MANAGEMENT’S LIQUIDITY PLANS
As of December 31, 2013, the Company had working capital and a stockholders’ deficiency of $143,095 and $29,600, respectively. During the year ended December 31, 2013, the Company incurred a net loss of $872,641. The Company has not generated any revenues and has incurred net losses since inception. These conditions raise substantial doubt about the Company’s ability to continue as a going concern.
As further discussed in Note 10, subsequent to December 31, 2013, the Company raised $2,600,000 from the sale of preferred stock, of which $210,000 was from the conversion of convertible promissory notes. The Company recognizes it will need to raise additional capital in order to meet its obligations and execute its business plan for at least the next twelve month period. There is no assurance that additional financing will be available when needed or that management will be able to obtain such financing on terms acceptable to the Company and that the Company will become profitable and generate positive operating cash flow in the future. If the Company is unable to raise sufficient additional funds, it will have to develop and implement a plan to further extend payables, reduce overhead or scale back its business plan until sufficient additional capital is raised to support further operations. There can be no assurance that such a plan will be successful.
Accordingly, the accompanying financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America, which contemplate continuation of the Company as a going concern and the realization of assets and the satisfaction of liabilities in the normal course of business. The carrying amounts of assets and liabilities presented in the financial statements do not necessarily represent realizable or settlement values. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
F-7
COHBAR, INC.
NOTES TO FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2013, 2012 AND 2011
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
USE OF ESTIMATES
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent liabilities at dates of the financial statements and the reported amounts of revenue and expenses during the periods. Actual results could differ from these estimates. The Company’s significant estimates and assumptions include the fair value of the Company’s stock, stock-based compensation, debt discount and the valuation allowance relating to the Company’s deferred tax assets.
CONCENTRATIONS OF CREDIT RISK
The Company maintains deposits in a financial institution which is insured by the Federal Deposit Insurance Corporation (“FDIC”). At various times, the Company has deposits in this financial institution in excess of the amount insured by the FDIC.
CASH
The Company considers all highly liquid investments with an original maturity of three months or less when purchased to be cash equivalents. As of December 31, 2013, 2012 and 2011, the Company did not have any cash equivalents. The Company includes as part of Restricted Cash any assets which are contractually restricted. Restricted Cash as of December 31, 2013 relates to proceeds received from a grant which is restricted to only certain activities of the Company (see Note 5).
PROPERTY AND EQUIPMENT
Property and equipment are stated at cost. Depreciation of computer and lab equipment is computed by use of the straight-line method based on the estimated useful lives of the assets, which range from one to five years. Expenditures for maintenance and repairs that do not improve or extend the expected lives of the assets are expensed to operations, while expenditures for major upgrades to existing items are capitalized. Upon retirement or other disposition of these assets, the costs and accumulated depreciation and amortization are removed from the accounts and resulting gains or losses are reflected in the results of operations.
IMPAIRMENT OF LONG-LIVED ASSETS
The Company reviews for the impairment of long-lived assets on an annual basis or whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. An impairment loss would be recognized when estimated future cash flows expected to result from the use of the asset and its eventual disposition is less than its carrying amount. The Company has not identified any such impairment losses.
FAIR VALUE OF FINANCIAL INSTRUMENTS
The Company measures the fair value of financial assets and liabilities based on the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most
F-8
COHBAR, INC.
NOTES TO FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2013, 2012 AND 2011
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
FAIR VALUE OF FINANCIAL INSTRUMENTS (CONTINUED)
advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. The Company utilizes three levels of inputs that may be used to measure fair value:
Level 1 – quoted prices in active markets for identical assets or liabilities
Level 2 – quoted prices for similar assets and liabilities in active markets or inputs that are observable
Level 3 – inputs that are unobservable (for example, cash flow modeling inputs based on assumptions)
The carrying amounts of cash, accounts payable, accrued liabilities and debt approximate fair value due to the short-term nature of these instruments.
COMMON STOCK PURCHASE WARRANTS
The Company classifies as equity any contracts that (i) require physical settlement or net-share settlement or (ii) provides the Company with a choice of net-cash settlement or settlement in its own shares (physical settlement or net-share settlement) providing that such contracts are indexed to the Company’s own stock. The Company classifies as assets or liabilities any contracts that (i) require net-cash settlement (including a requirement to net cash settle the contract if an event occurs and if that event is outside the Company’s control), or (ii) gives the counterparty a choice of net-cash settlement or settlement in shares (physical settlement or net-share settlement). The Company assesses classification of its common stock purchase warrants and other free standing derivatives at each reporting date to determine whether a change in classification between assets and liabilities is required. The Company’s free standing derivatives consist of warrants to purchase common stock that were issued in connection with its notes payable. The Company evaluated these warrants to assess their proper classification using the applicable criteria enumerated under U.S. GAAP and determined that the common stock purchase warrants meet the criteria for equity classification in the balance sheet as of December 31, 2013. There were no warrants outstanding as of December 31, 2012 and 2011.
INCOME TAXES
The Company recognizes deferred tax assets and liabilities for the expected future tax consequences of items that have been included or excluded in the financial statements or tax returns. Deferred tax assets and liabilities are determined on the basis of the difference between the tax basis of assets and liabilities and their respective financial reporting amounts (“temporary differences”) at enacted tax rates in effect for the years in which the temporary differences are expected to reverse.
Management has evaluated and concluded that there were no material uncertain tax positions requiring recognition in the Company’s financial statements as of December 31, 2013, 2012 and 2011. The Company does not expect any significant changes in the unrecognized tax benefits within twelve months of the reporting date.
The Company classifies interest expense and any related penalties related to income tax uncertainties as a component of income tax expense. No interest or penalties have been recognized during the years ended December 31, 2013, 2012 and 2011.
F-9
COHBAR, INC.
NOTES TO FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2013, 2012 AND 2011
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
RESEARCH AND DEVELOPMENT EXPENSES
The Company expenses all research and development expenses as incurred. These costs include payroll, employee benefits, supplies, contracted for lab services, depreciation and other personnel-related costs associated with product development.
SHARE-BASED PAYMENT
The Company accounts for share-based payments using the fair value method. For employees and directors, the fair value of the award is measured, as discussed below, on the grant date. For non-employees, fair value is generally valued based on the fair value of the services provided or the fair value of the common stock on the measurement date, whichever is more readily determinable and re-measured on interim financial reporting dates until the service is complete. The Company has granted stock options at exercise prices no less than the fair market value as determined by the board of directors, with input from management.
The weighted-average fair value of options and warrants has been estimated on the date of grant using the Black-Scholes pricing model. The fair value of each instrument is estimated on the date of grant utilizing certain assumptions for a risk free interest rate, volatility and expected remaining lives of the awards. Since shares of the Company have not been publicly traded, the fair value of stock-based payment awards was estimated using a volatility derived from an index of comparable entities. The assumptions used in calculating the fair value of share-based payment awards represent management’s best estimates, but these estimates involve inherent uncertainties and the application of management judgment. As a result, if factors change and the Company uses different assumptions, the Company’s stock-based compensation expense could be materially different in the future. In addition, the Company is required to estimate the expected forfeiture rate and only recognize expense for those shares expected to vest. In estimating the Company’s forfeiture rate, the Company analyzed its historical forfeiture rate, the remaining lives of unvested options, and the number of vested options as a percentage of total options outstanding. If the Company’s actual forfeiture rate is materially different from its estimate, or if the Company reevaluates the forfeiture rate in the future, the stock-based compensation expense could be significantly different from what the Company has recorded in the current period.
The weighted-average Black-Scholes assumptions are as follows:
| For The Years Ended December 31, | ||||||
| 2013 | 2012 |
2011 | ||||
| Expected life |
10 years | 6 years | N/A | |||
| Risk free interest rate |
1.86% | 2.22% | N/A | |||
| Expected volatility |
138% | 138% | N/A | |||
| Expected dividend yield |
0% | 0% | N/A | |||
As of December 31, 2013, total unrecognized stock option compensation expense is $3,057, which will be recognized as those options vest over a period of approximately three years. The amount of future stock option compensation expense could be affected by any future option grants or by any option holders leaving the Company before their grants are fully vested.
F-10
COHBAR, INC.
NOTES TO FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2013, 2012 AND 2011
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
NET LOSS PER SHARE OF COMMON STOCK
Basic net loss per share is computed by dividing net loss attributable to common stockholders by the weighted average number of common shares outstanding during the period. Diluted net loss per share reflects the potential dilution that could occur if securities or other instruments to issue common stock were exercised or converted into common stock. Potentially dilutive securities are excluded from the computation of diluted net loss per share as their inclusion would be anti-dilutive and consist of the following:
| December 31, | December 31, | December 31, | ||||||||||
| 2013 | 2012 | 2011 | ||||||||||
| Preferred Stock Series A |
— | — | 1,012,968 | |||||||||
| Warrants |
15,596 | — | — | |||||||||
| Options |
163,971 | 1,471,699 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Totals |
179,567 | 1,471,699 | 1,012,968 | |||||||||
|
|
|
|
|
|
|
|||||||
RECENT ACCOUNTING PRONOUNCEMENTS
In June 2014, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2014-10, “Development Stage Entities (Topic 915): Elimination of Certain Financial Reporting Requirements, Including an Amendment to Variable Interest Entities Guidance in Topic 810, Consolidation.” This ASU removes the definition of a development stage entity from the ASC, thereby removing the financial reporting distinction between development stage entities and other reporting entities from GAAP. In addition, the ASU eliminates the requirements for development stage entities to (1) present inception-to-date information in the statements of operations, cash flows, and shareholders’ deficit, (2) label the financial statements as those of a development stage entity, (3) disclose a description of the development stage activities in which the entity is engaged, and (4) disclose in the first year in which the entity is no longer a development stage entity that in prior years it had been in the development stage. ASU 2014-10 is effective for annual periods beginning after December 15, 2014. ASU 2014-10 does allow early adoption for entity’s that have not yet issued financial statements. The Company has early adopted ASU 2014-10 and reflected this adoption in its financial statement presentation contained herein.
The FASB has issued ASU No. 2014-12, Compensation – Stock Compensation (Topic 718): Accounting for Share-Based Payments When the Terms of an Award Provide That a Performance Target Could Be Achieved after the Requisite Service Period. This ASU requires that a performance target that affects vesting, and that could be achieved after the requisite service period, be treated as a performance condition. As such, the performance target should not be reflected in estimating the grant date fair value of the award. This update further clarifies that compensation cost should be recognized in the period in which it becomes probable that the performance target will be achieved and should represent the compensation cost attributable to the period(s) for which the requisite service has already been rendered. The amendments in this ASU are effective for annual periods and interim periods within those annual periods beginning after December 15, 2015. Earlier adoption is permitted. The adoption of this standard is not expected to have a material impact on the Company’s financial position and results of operations.
Recent accounting standards that have been issued or proposed by the FASB or other standards-setting bodies that do not require adoption until a future a date are not expected to have a material impact on the Company’s financial statements upon adoption.
F-11
COHBAR, INC.
NOTES TO FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2013, 2012 AND 2011
NOTE 4 – PROPERTY AND EQUIPMENT
Property and equipment consist of the following:
| December 31, 2013 |
December 31, 2012 |
December 31, 2011 |
||||||||||
| Computer and equipment |
$ | 5,396 | $ | 4,915 | $ | 4,915 | ||||||
| Lab equipment |
3,496 | 3,496 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Totals |
8,892 | 8,411 | 4,915 | |||||||||
| Less: accumulated depreciation |
(4,283 | ) | (2,390 | ) | (227 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Property and equipment, net |
$ | 4,609 | $ | 6,021 | $ | 4,688 | ||||||
|
|
|
|
|
|
|
|||||||
Depreciation and amortization expense related to property and equipment for the years ended December 31, 2013, 2012 and 2011 was $2,266, $2,163 and $227, respectively.
NOTE 5 – NOTE PAYABLE
In 2013, the Company was awarded a grant from the Alzheimer’s Drug Discovery Foundation. The award was paid in two installments of $102,630 totaling $205,260. The Company executed Promissory Notes (the “Notes”) which governed the terms of the repayment of the grant. The Notes have a term of 4 years and are due and payable in 2017 unless there is a change of control, as defined. In the event of a change of control, the total principal amount that is outstanding under the Notes, plus all accrued and unpaid interest become immediately due and payable. The Notes include interest rates that are equal to the prime rate that is two days prior to the date of the Notes (3.25% at December 31, 2013). In connection with the grant award the Company also issued to the Alzheimer’s Drug Discovery Foundation a warrant to purchase 15,596 shares of the Company’s common stock at an exercise price of $0.99. The Company determined the fair value of the warrants issued using the Black-Scholes pricing model with the assumptions discussed in Note 3, and allocated the proceeds based on the relative fair value of the debt instrument and the related warrants. The aggregate deferred debt discount related to the Note was $785. The Company amortized $138 of the debt discount during the year ended December 31, 2013. The warrant expires on the 10 year anniversary of the grant date.
NOTE 6 – COMMITMENTS AND CONTINGENCIES
LITIGATIONS, CLAIMS AND ASSESSMENTS
The Company may be involved in legal proceedings, claims and assessments arising in the ordinary course of business. Such matters are subject to many uncertainties, and outcomes are not predictable with assurance. There are no such matters that are included in the financial statements as of December 31, 2013.
LICENSING AGREEMENTS
Effective November 30, 2011, the Company entered into an Exclusive License Agreement (the “2011 Exclusive Agreement”) with the Regents of the University of California (the “Regents”) whereby the Regents granted to the Company an exclusive license for the use of certain patents. The Company paid the Regents an initial license issue fee of $35,000, which was charged to General and Administrative expense, as incurred. The Company agreed to pay the licensors specified development milestone payments
F-12
COHBAR, INC.
NOTES TO FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2013, 2012 AND 2011
NOTE 6 – COMMITMENTS AND CONTINGENCIES (CONTINUED)
LICENSING AGREEMENTS (CONTINUED)
aggregating up to $765,000 for the first product sold under the license. Milestone payments for additional products developed and sold under the license are reduced by 50%. The Company is also required to pay annual maintenance fees to the licensors. Aggregate maintenance fees for the first five years following execution of the agreement are $80,000. Thereafter, the Company is required to pay maintenance fees of $50,000 annually until the first sale of a licensed product. In addition, for the duration of the 2011 Exclusive Agreement, the Company is required to pay the licensors royalties equal to 2% of its worldwide net sales of drugs, therapies or other products developed from claims covered by the licensed patents, subject to a minimum royalty payment of $75,000 annually, beginning after the first commercial sale of a licensed product. The Company is required to pay royalties ranging from 8% of worldwide sublicense sales of covered products (if the sublicense is entered after commencement of phase II clinical trials to 12% of worldwide sublicense sales (if the sublicense is entered prior to commencement of phase I clinical trials). The agreement also requires the Company to meet certain diligence and development milestones, including filing of an Investigational New Drug (“IND”) Application for a product covered by the agreement on or before the seventh anniversary of the agreement date. Through December 31, 2013, no royalties have been incurred under the agreement.
Effective August 6, 2013, the Company entered into an Exclusive License Agreement (the “2013 Exclusive Agreement”) with the Regents whereby the Regents granted to the Company an exclusive license for the use of certain other patents. The Company paid Regents an initial license issue fee of $10,000 for these other patents, which was charged to General and Administrative expense, as incurred. The Company agreed to pay the Regents specified development milestone payments aggregating up to $765,000 for the first product sold under the 2013 Exclusive Agreement. Milestone payments for additional products developed and sold under the 2013 Exclusive Agreement are reduced by 50%. In addition, for the duration of the 2013 Exclusive Agreement, the Company is required to pay the Regents royalties equal to 2% of the Company’s worldwide net sales of drugs, therapies or other products developed from claims covered by the licensed patent, subject to a minimum royalty payment of $75,000 annually, beginning after the first commercial sale of a licensed product.
The Company is required to pay the Regents royalties ranging from 8% of worldwide sublicense sales of covered products (if the sublicense is entered after commencement of phase II clinical trials to 12% of worldwide sublicense sales (if the sublicense is entered prior to commencement of phase I clinical trials). The agreement also requires the Company to meet certain diligence and development milestones, including filing of an IND Application for a product covered by the agreement on or before the seventh anniversary of the agreement date. Through December 31, 2013, no royalties have been incurred under the agreement.
OPERATING LEASE
The Company rents lab space on a month to month basis in Pasadena, California. Rent expense amounted to $25,200, $24,600 and $6,800 for the years ended December 31, 2013, 2012 and 2011, respectively.
F-13
COHBAR, INC.
NOTES TO FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2013, 2012 AND 2011
NOTE 7 – INCOME TAXES
The tax effects of temporary differences that give rise to deferred tax assets as of December 31, 2013, 2012 and 2011 are presented below:
| For the Years Ended December 31, | ||||||||||||
| 2013 | 2012 | 2011 | ||||||||||
| Deferred Tax Assets: |
||||||||||||
| Net operating loss carryforward |
$ | 1,031,451 | $ | 690,102 | $ | 116,257 | ||||||
| Research and development credit carryforward |
10,826 | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total deferred tax asset |
1,042,277 | 690,102 | 116,257 | |||||||||
| Valuation allowance |
(1,042,277 | ) | (690,102 | ) | (116,257 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Deferred tax asset, net of valuation allowance |
$ | — | $ | — | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
A reconciliation of the statutory federal income tax rate to the Company’s effective tax rate is as follows:
| For the Years Ended December 31, | ||||||||||||
| 2013 | 2012 | 2011 | ||||||||||
| U.S. statutory federal rate |
(34.0 | )% | (34.0 | )% | (34.0 | )% | ||||||
| State income taxes, net of federal tax benefit |
(5.9 | )% | (5.9 | )% | (5.9 | )% | ||||||
| Permanent differences |
0.8 | % | 0.9 | % | 0.2 | % | ||||||
| Change in valuation allowance |
39.1 | % | 39.0 | % | 39.7 | % | ||||||
|
|
|
|
|
|
|
|||||||
| Income tax provision (benefit) |
— | % | — | % | — | % | ||||||
|
|
|
|
|
|
|
|||||||
The income tax provision consists of the following:
| For the Years Ended December 31, | ||||||||||||
| 2013 | 2012 | 2011 | ||||||||||
| Federal |
||||||||||||
| Current |
$ | — | $ | — | $ | — | ||||||
| Deferred |
(294,558 | ) | (488,991 | ) | (98,610 | ) | ||||||
| State and local |
||||||||||||
| Current |
— | — | ||||||||||
| Deferred |
(51,114 | ) | (84,854 | ) | (17,112 | ) | ||||||
| Change in valuation allowance |
345,672 | 573,845 | 115,722 | |||||||||
|
|
|
|
|
|
|
|||||||
| Income tax provision (benefit) |
$ | — | $ | — | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
The Company assesses the likelihood that deferred tax assets will be realized. To the extent that realization is not more likely than not, a valuation allowance is established. Based upon the Company’s losses since inception, management believes that it is more-likely-than-not that future benefits of deferred tax assets will not be realized.
The Company files income tax returns in the U.S. federal jurisdiction and various state jurisdictions, principally California and New Jersey. The Company is subject to examination by the various taxing authorities. The Company’s federal and state income tax returns for tax years beginning in 2010 remain subject to examination.
F-14
COHBAR, INC.
NOTES TO FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2013, 2012 AND 2011
NOTE 7 – INCOME TAXES (CONTINUED)
At December 31, 2013, 2012 and 2011, the Company had approximately $2,582,501, $1,727,848 and $291,079, respectively, of federal and state net operating loss carryovers that may be available to offset future taxable income. The net operating loss carry forwards, if not utilized, will expire from 2029 to 2033 for federal and state purposes. In accordance with Section 382 of the Internal Revenue Code, the usage of the Company’s net operating loss carryforward could be limited in the event of a change in ownership.
NOTE 8 – STOCKHOLDERS’ DEFICIENCY
AUTHORIZED CAPITAL
As of December, 31, 2013, the Company has authorized the issuance and sale of up to 9,780,000 shares of capital stock consisting of 7,000,000 shares of common stock having a par value of $0.001 and 2,780,000 shares of Preferred Stock having a par value of $0.001 per share (see Note 10). The annual dividend rate is 6% per share for Series A Preferred Stock. The holders of Preferred Stock are entitled to receive dividends, when, as and if declared by the Company’s Board of Directors. The dividends on shares of Preferred Stock are cumulative.
As of December 31, 2013, there were no declared but unpaid dividends or undeclared dividend arrearages on any shares of the Company’s capital stock. The holders of Preferred Stock are entitled to be paid out of the assets of the corporation before any payment is made to the holders of common stock in the event of any voluntary or involuntary liquidations, dissolution or winding up of the Company. The Company has reserved 2,251,041 shares of its common stock for the issuance of stock options and restricted stock to its employees, officers, directors and consultants.
PREFERRED STOCK
During the year ended December 31, 2011, the Company issued 1,012,968 shares of Series A Preferred Stock in the amount of $1,000,000, inclusive of issuance costs of $194,278.
During the year ended December 31, 2012, the Company issued 1,772,694 shares of Series A Preferred Stock in the amount of $1,750,000, inclusive of issuance costs of $793. The Series A Preferred Stock were issued to one investor who committed to funding the Company an aggregate of $10,000,000 in a series of agreed upon purchases. The investor failed to meet this obligation which created an automatic conversion of the Preferred Stock to Common Stock. During the year ended December 31, 2012, 2,785,662 shares of Series A Preferred Stock were converted to Common Stock. At the time of conversion, there were no declared but unpaid or undeclared dividends relating to the Series A Preferred Stock. In 2013, two stockholders purchased all 2,785,662 shares of common stock held by the investor pursuant to a Stock Purchase and Sale Agreement among the three parties.
STOCK OPTIONS
The Company has one incentive stock plan, the 2011 Equity Incentive Plan (the “2011 Plan”). The Company has granted stock options to employees, non-employee directors and consultants from the 2011 Plan through the year ended December 31, 2013. Options granted under the Plan may be Incentive Stock Options or Non-statutory Stock Options, as determined by the Administrator at the time of grant. At December 31, 2013, 2,087,072 shares of the Company’s common stock were available for future issuance under the 2011 Plan.
F-15
COHBAR, INC.
NOTES TO FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2013, 2012 AND 2011
NOTE 8 – STOCKHOLDERS’ DEFICIENCY (CONTINUED)
STOCK OPTIONS (CONTINUED)
The Company recorded $16,207, $29,910 and $0 of stock based compensation in the years ended December 31, 2013, 2012 and 2011, respectively. The compensation expense associated with stock-based awards granted to individuals is recorded by the Company in the same expense classifications as the cash compensation paid to those same individuals.
During the year ended December 31, 2012, the Company issued an aggregate of 1,471,699 stock options to employees and consultants with an exercise price of $0.05 and a fair value of $0.05 per share. The stock options granted in 2012 are subject to vesting over three to four years and have a term of ten years.
During the year ended December 31, 2013, the Company cancelled 1,307,728 options due to the termination of employees. The cancelled options were added back to the available pool for future issuance.
The following table represents stock option activity for the years ended December 31, 2013, 2012 and 2011:
| Weighted Average | ||||||||||||||||||||||||||||
| Stock Options | Exercise Price | Fair Value Vested |
Contractual Life (Years) |
Aggregate Intrinsic Value |
||||||||||||||||||||||||
| Outstanding | Exercisable | Outstanding | Exercisable | |||||||||||||||||||||||||
| Balance – December 31, 2010 |
— | — | $ | — | ||||||||||||||||||||||||
| Granted |
— | — | ||||||||||||||||||||||||||
| Exercised |
— | — | ||||||||||||||||||||||||||
| Cancelled |
— | — | ||||||||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||||||
| Balance – December 31, 2011 |
— | — | $ | — | ||||||||||||||||||||||||
| Granted |
1,471,699 | 0.05 | ||||||||||||||||||||||||||
| Exercised |
— | — | ||||||||||||||||||||||||||
| Cancelled |
— | — | ||||||||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||||||
| Balance – December 31, 2012 |
1,471,699 | 332,861 | $ | 0.05 | $ | 0.05 | $ | 0.05 | 9.26 | |||||||||||||||||||
| Granted |
— | — | ||||||||||||||||||||||||||
| Exercised |
— | — | ||||||||||||||||||||||||||
| Cancelled |
(1,307,728 | ) | 0.05 | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance – December 31, 2013 |
163,971 | 83,123 | $ | 0.05 | $ | 0.05 | $ | 0.05 | 8.26 | $ | 34,434 | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
The following table summarizes information on stock options outstanding and exercisable as of December 31, 2013:
| Exercise Price |
Number Outstanding |
Weighted Average Remaining Contractual Term |
Weighted Average Exercise Price |
Number Exercisable |
Weighted Average Exercise Price |
|||||||||||||||
| $ 0.05 |
163,971 | 8.26 | $ | 0.05 | 83,123 | $ | 0.05 | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Totals |
163,971 | 83,123 | ||||||||||||||||||
|
|
|
|
|
|||||||||||||||||
F-16
COHBAR, INC.
NOTES TO FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2013, 2012 AND 2011
NOTE 8 – STOCKHOLDERS’ DEFICIENCY (CONTINUED)
WARRANTS
The following table represents warrant activity for the years ended December 31, 2013, 2012 and 2011:
| Weighted Average | ||||||||||||||||||||||||
| Warrants | Exercise Price | Fair Value Vested |
Contractual Life (Years) |
|||||||||||||||||||||
| Outstanding | Exercisable | Outstanding | Exercisable | |||||||||||||||||||||
| Balance – December 31, 2010 |
— | — | $ | — | ||||||||||||||||||||
| Granted |
— | — | ||||||||||||||||||||||
| Exercised |
— | — | ||||||||||||||||||||||
| Cancelled |
— | — | ||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Balance – December 31, 2011 |
— | — | $ | — | ||||||||||||||||||||
| Granted |
— | — | ||||||||||||||||||||||
| Exercised |
— | — | ||||||||||||||||||||||
| Cancelled |
— | — | ||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Balance – December 31, 2012 |
— | — | $ | — | ||||||||||||||||||||
| Granted |
15,596 | 15,596 | 0.99 | |||||||||||||||||||||
| Exercised |
— | — | ||||||||||||||||||||||
| Cancelled |
— | — | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance – December 31, 2013 |
15,596 | 15,596 | $ | 0.99 | $ | 0.99 | $ | 0.05 | — | |||||||||||||||
NOTE 9 – RELATED PARTY
During the years ended December 31, 2013, 2012 and 2011, the Company paid the wife of a stockholder an aggregate of $10,500, $7,000 and $3,500, respectively, for consulting services. The consulting services provided related to accounting activities.
NOTE 10 – SUBSEQUENT EVENTS
In January 2014, the Company issued Convertible Promissory Notes for cash proceeds of $210,000 (“January 2014 Notes”). The January 2014 Notes had a maturity date of one year, interest of 0% and included a warrant to purchase an aggregate of 20,946 shares of the Company’s Common Stock at an exercise price of $0.50 per share. The warrants expire the earlier of a liquidation event, upon the effective date of the Company’s initial public offering or in one year. If the January 2014 Notes were not repaid or converted on or prior to the date that is six months after the issuance, the Company was required to issue to the holders of the January 2014 Notes additional warrants equal to the amount of the initial warrants issued. The Company determined the fair value of the warrants issued using the Black-Scholes pricing model, and allocated the proceeds based on the relative fair value of the debt instruments and the related warrants. The aggregate deferred debt discount related to the January 2014 Notes was $137. In April 2014, the January 2014 Notes were converted to shares of the Series B Preferred Stock.
In April 2014, the Company amended its Certificate of Incorporation to increase to the total number of authorized shares of common stock. The Company, following the amendment, has authorized the issuance and sale of up to 45,000,000 shares of stock, consisting of 37,000,000 shares of common stock having a par value of $0.001 and 8,000,000 shares of Preferred Stock having a par value of $0.001 per share.
F-17
COHBAR, INC.
NOTES TO FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2013, 2012 AND 2011
NOTE 10 – SUBSEQUENT EVENTS (CONTINUED)
Between April 2014 and June 2014, the Company issued 5,200,000 shares of Series B Preferred Stock in the amount of $2,600,000, net of issuance costs of $65,018. 420,000 shares of the Series B Preferred Stock in the amount of $210,000 were issued upon the conversion of the January 2014 Notes. The Series B Preferred Stock has a par value of $0.001 and was issued at $0.50 per share. The purchasers of Series B Preferred Stock entered into put agreements requiring the purchasers, at the Company’s option, to purchase from the Company securities of the same type as those sold to investors in any future public offering of the Company’s securities, at the same price as the securities sold in the initial public offering, for an aggregate purchase price of up to $2,600,000. The put agreements expire upon the first occurrence of a change in control or in three years. The Company can exercise its rights under the put agreements beginning on the date the Company first submits an IPO Registration Statement for review by the Securities and Exchange Commission and ending the earlier of the day that is 21 days prior to the effective date of the IPO Registration or the expiration date of the put agreements. Since the rights under the put agreements are contingent on the filing of an IPO Registration Statement, it cannot be valued as of its grant date. As of December 31, 2013, the Company had incurred Deferred Offering Costs related to this issuance aggregating $26,209.
In April 2014, the Company granted 1,061,248 stock options to directors, employees and consultants with an exercise price of $0.26 and a fair value of $0.18 per option. The stock options contained vesting schedules that ranged from two to four years and have a term of ten years.
In April 2014, the Company issued 797,075 warrants to its Chief Executive Officer. The warrants have an exercise price of $0.26 and a fair value of $0.21 per warrant. The warrants expire on the earlier of a liquidation event, as defined, or in ten years.
In July 2014, the Company issued 100,000 warrants to consultants. The warrants have an exercise price of $0.26 and a fair value of $0.17 per warrant. The warrants expire on the earlier of a liquidation event, as defined, or in five years.
Management has evaluated subsequent events to determine if events or transactions occurring through August 6, 2014, the date on which the financial statements were available to be issued require adjustment or disclosure in the Company’s financial statements.
F-18
Cohbar, Inc.
| September 30, 2014 |
December 31, 2013 |
|||||||
| (unaudited) | ||||||||
| ASSETS | ||||||||
| Current assets: |
||||||||
| Cash |
$ | 1,818,843 | $ | 145,170 | ||||
| Restricted cash |
4,055 | 126,195 | ||||||
| Prepaid expenses and other current assets |
30,150 | 15,124 | ||||||
|
|
|
|
|
|||||
| Total current assets |
1,853,048 | 286,489 | ||||||
| Property and equipment, net |
2,735 | 4,609 | ||||||
| Deferred offering costs |
247,013 | 26,209 | ||||||
| Other assets |
1,100 | 1,100 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 2,103,896 | $ | 318,407 | ||||
|
|
|
|
|
|||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIENCY) | ||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 97,515 | $ | 54,645 | ||||
| Accrued liabilities |
259,177 | 69,635 | ||||||
| Accrued payroll and other compensation |
42,876 | 19,114 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
399,568 | 143,394 | ||||||
| Note payable, net of debt discount of $500 and $647 as of September 30, 2014 and December 31, 2013, respectively |
204,760 | 204,613 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
604,328 | 348,007 | ||||||
|
|
|
|
|
|||||
| Commitments and contingencies |
||||||||
| Stockholders’ equity (deficiency) |
||||||||
| Preferred stock, $0.001 par value, Authorized- 8,000,000 shares; |
||||||||
| Issued and outstanding as of September 30, 2014 and December 31, 2013 as follows: |
||||||||
| Preferred stock – Series A – issued and outstanding 0 shares as of September 30, 2014 and December 31, 2013, respectively |
— | — | ||||||
| Convertible preferred stock – Series B – issued and outstanding 5,400,000 shares as of September 30, 2014 and 0 as of December 31, 2013 |
5,400 | — | ||||||
| Common stock, $0.001 par value, Authorized – 37,000,000 shares; |
||||||||
| Issued and outstanding 12,915,343 shares as of September 30, 2014 and December 31, 2013 |
12,915 | 12,915 | ||||||
| Additional paid-in capital |
5,442,495 | 2,594,128 | ||||||
| Accumulated deficit |
(3,961,242 | ) | (2,636,643 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ equity (deficiency) |
1,499,568 | (29,600 | ) | |||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ equity (deficiency) |
$ | 2,103,896 | $ | 318,407 | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these condensed financial statements
F-19
Cohbar, Inc.
Condensed Statements of Operations
(unaudited)
| Three Months Ended September 30 |
Nine Months Ended September 30, |
|||||||||||||||
| 2014 | 2013 | 2014 | 2013 | |||||||||||||
| Revenues |
$ | — | $ | — | $ | — | $ | — | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
159,883 | 103,210 | 405,215 | 376,272 | ||||||||||||
| General and administrative |
246,182 | 64,321 | 914,399 | 259,639 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
406,065 | 167,531 | 1,319,614 | 635,911 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Operating loss |
(406,065 | ) | (167,531 | ) | (1,319,614 | ) | (635,911 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Other income (expense): |
||||||||||||||||
| Interest income |
193 | 136 | 440 | 447 | ||||||||||||
| Interest expense |
(1,700 | ) | (862 | ) | (5,141 | ) | (2,335 | ) | ||||||||
| Amortization of debt discount |
(49 | ) | — | (284 | ) | (82 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total other income (expense) |
(1,556 | ) | (726 | ) | (4,985 | ) | (1,970 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | (407,621 | ) | $ | (168,257 | ) | $ | (1,324,599 | ) | $ | (637,881 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Basic and diluted net loss per share |
$ | (0.03 | ) | $ | (0.01 | ) | $ | (0.10 | ) | $ | (0.05 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Weighted average common shares outstanding – |
12,915,343 | 12,915,343 | 12,915,343 | 12,915,343 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The accompanying notes are an integral part of these condensed financial statements
F-20
Cohbar, Inc.
Condensed Statements of Cash Flows
(unaudited)
| For The Nine Months Ended September 30, | ||||||||
| 2014 | 2013 | |||||||
| Cash flows from operating activities: |
||||||||
| Net loss |
$ | (1,324,599 | ) | $ | (637,881 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
| Depreciation and amortization |
1,874 | 1,675 | ||||||
| Loss on disposal of property and equipment |
— | 334 | ||||||
| Stock-based compensation |
239,760 | 15,800 | ||||||
| Amortization of debt discount |
284 | 82 | ||||||
| Changes in operating assets and liabilities: |
||||||||
| Restricted cash |
122,140 | 46,291 | ||||||
| Prepaid expenses and other current assets |
(15,026 | ) | 3,171 | |||||
| Accounts payable |
42,870 | 1,264 | ||||||
| Accrued liabilities |
189,542 | 12,432 | ||||||
| Accrued payroll and other compensation |
23,762 | (4,133 | ) | |||||
|
|
|
|
|
|||||
| Net cash used in operating activities |
(719,393 | ) | (560,965 | ) | ||||
|
|
|
|
|
|||||
| Cash flows from investing activities: |
||||||||
| Restricted cash |
— | (205,260 | ) | |||||
|
|
|
|
|
|||||
| Net cash used in investing activities |
— | (205,260 | ) | |||||
|
|
|
|
|
|||||
| Cash flows from financing activities: |
||||||||
| Deferred offering costs |
(247,013 | ) | — | |||||
| Proceeds from the issuance of converible preferred stock, net |
2,430,079 | — | ||||||
| Proceeds from convertible notes |
210,000 | — | ||||||
| Proceeds from note payable |
— | 204,475 | ||||||
|
|
|
|
|
|||||
| Net cash provided by financing activities |
2,393,066 | 204,475 | ||||||
|
|
|
|
|
|||||
| Net increase (decrease) in cash |
1,673,673 | (561,750 | ) | |||||
| Cash at beginning of period |
145,170 | 878,094 | ||||||
|
|
|
|
|
|||||
| Cash at end of period |
$ | 1,818,843 | $ | 316,344 | ||||
|
|
|
|
|
|||||
| Non-cash investing and financing activities: |
||||||||
| Warrants issued in connection with note payable |
$ | — | $ | 785 | ||||
| Warrants issued in connection with bridge loans |
$ | 137 | $ | — | ||||
| Conversion of convertible notes to Series B Preferred Stock |
$ | 210,000 | $ | — | ||||
The accompanying notes are an integral part of these condensed financial statements
F-21
COHBAR, INC.
NOTES TO CONDENSED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2014
(unaudited)
NOTE 1 – BUSINESS ORGANIZATION AND NATURE OF OPERATIONS
Cohbar, Inc. (“Cohbar” or the “Company”) is a research stage biotechnology company focused on the discovery and development of novel peptide-based therapeutics for the treatment of diseases with significant unmet need. The Company’s research focuses specifically on the biology of Mitochondrial-Derived Peptides, or MDPs. To date, the Company has conducted investigational research into MDPs to evaluate their therapeutic potential and has identified four MDPs for possible advancement into drug candidate programs targeting one or more indications from a variety of diseases, including cancer, Alzheimer’s disease, atherosclerosis and certain metabolic disorders such as Type 2 diabetes and obesity.
The Company’s primary activities since inception have been the development and implementation of its business plans, negotiating inbound intellectual property licenses and other agreements, raising capital and conducting research on its MDPs. To date, the Company has not generated any revenues from operations and does not expect to generate any revenues in the near future.
The accompanying unaudited condensed financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) for interim financial information. Accordingly, they do not include all of the information and disclosures required by U.S. GAAP for annual financial statements. In the opinion of management, such statements include all adjustments (consisting only of normal recurring items) that are considered necessary for a fair presentation of the condensed financial statements of the Company as of September 30, 2014 and for the three and nine months ended September 30, 2014 and 2013. The results of operations for the three and nine months ended September 30, 2014 and 2013 are not necessarily indicative of the operating results for the full year ending December 31, 2014. These condensed financial statements should be read in conjunction with the audited financial statements and related disclosures of the Company as of December 31, 2013 and for the year then ended, which are included elsewhere in this document.
In April 2014, the Company effected a 3.6437695-for-1 stock split of its issued and outstanding shares of common stock. All references in these condensed financial statements to the number of shares, options and other common stock equivalents, price per share and weighted average number of shares outstanding of common stock have been adjusted to retroactively reflect the effect of the stock split.
NOTE 2 – GOING CONCERN AND MANAGEMENT’S LIQUIDITY PLANS
As of September 30, 2014, the Company had working capital and a stockholders’ equity of $1,453,480 and $1,499,568, respectively. During the nine months ended September 30, 2014, the Company incurred a net loss of $1,324,599. The Company has not generated any revenues, has incurred net losses since inception and does not expect to generate revenues in the near term. These conditions raise substantial doubt about the Company’s ability to continue as a going concern.
The Company recognizes it will need to raise additional capital in order to meet its obligations and execute its business plan for at least the next twelve month period. There is no assurance that additional financing will be available when needed or that management will be able to obtain such financing on terms acceptable to the Company and that the Company will become profitable and generate positive operating cash flow in the future. If the Company is unable to raise sufficient additional funds, it will have to develop and implement a plan to further extend payables, reduce overhead or scale back its business plan until sufficient additional capital is raised to support further operations. There can be no assurance that such a plan will be successful.
F-22
COHBAR, INC.
NOTES TO CONDENSED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2014
(unaudited)
NOTE 2 – GOING CONCERN AND MANAGEMENT’S LIQUIDITY PLANS (CONTINUED)
Accordingly, the accompanying condensed financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America, which contemplate continuation of the Company as a going concern and the realization of assets and the satisfaction of liabilities in the normal course of business. The carrying amounts of assets and liabilities presented in the condensed financial statements do not necessarily represent realizable or settlement values. The condensed financial statements do not include any adjustments that might result from the outcome of this uncertainty.
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
USE OF ESTIMATES
The preparation of condensed financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent liabilities at dates of the financial statements and the reported amounts of revenue and expenses during the periods. Actual results could differ from these estimates. The Company’s significant estimates and assumptions include the fair value of the Company’s stock, stock-based compensation, debt discount and the valuation allowance relating to the Company’s deferred tax assets.
DEFERRED OFFERING COSTS
The Company classifies amounts related to a potential future financing not closed as of the balance sheet date as Deferred Offering Costs. As of September 30, 2014, the Company capitalized costs in the amount of $247,013 that relate to a potential future financing as Deferred Offering Costs in the accompanying condensed balance sheet.
SHARE-BASED PAYMENT
The Company accounts for share-based payments at fair value. For employees and directors, the fair value of the award is measured, as discussed below, on the grant date. For non-employees, fair value is generally valued based on the fair value of the services provided or the fair value of the common stock on the measurement date, whichever is more readily determinable and re-measured on interim financial reporting dates until the service is complete. The Company has granted stock options at exercise prices no less than the fair value as determined by the board of directors, with input from management.
The weighted-average fair value of options and warrants has been estimated on the date of grant using the Black-Scholes pricing model. The fair value of each instrument is estimated on the date of grant utilizing certain assumptions for a risk free interest rate, volatility and expected remaining lives of the awards. The risk free rate is based on the US Treasury rate at the time of the grant. Since shares of the Company have not been publicly traded, the fair value of stock-based payment awards was estimated using a volatility derived from an index of comparable entities. The assumptions used in calculating the fair value of share-based payment awards represent management’s best estimates, but these estimates involve inherent uncertainties and the application of management judgment. As a result, if factors change and the Company uses different assumptions, the Company’s stock-based compensation expense could be materially different in the future.
F-23
COHBAR, INC.
NOTES TO CONDENSED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2014
(unaudited)
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
SHARE-BASED PAYMENT (CONTINUED)
In addition, the Company is required to estimate the expected forfeiture rate and only recognize expense for those shares expected to vest. In estimating the Company’s forfeiture rate, the Company analyzed its historical forfeiture rate, the remaining lives of unvested options, and the number of vested options as a percentage of total options outstanding. If the Company’s actual forfeiture rate is materially different from its estimate, or if the Company reevaluates the forfeiture rate in the future, the stock-based compensation expense could be significantly different from what the Company has recorded in the current period.
The weighted-average Black-Scholes assumptions are as follows:
| For The Three And Nine Months Ended September 30, | ||||||
| 2014 | 2013 | |||||
| Expected life |
6 years | N/A | ||||
| Risk free interest rate |
2.42% | N/A | ||||
| Expected volatility |
80% | N/A | ||||
| Expected dividend yield |
0% | N/A | ||||
As of September 30, 2014, total unrecognized stock option compensation expense was $127,409 which will be recognized as those options vest over a period of approximately three years. The amount of future stock option compensation expense could be affected by any future option grants or by any option holders leaving the Company before their grants are fully vested.
NET LOSS PER SHARE OF COMMON STOCK
Basic net loss per share is computed by dividing net loss attributable to common stockholders by the weighted average number of common shares outstanding during the period. Diluted net loss per share reflects the potential dilution that could occur if securities or other instruments to issue common stock were exercised or converted into common stock. Potentially dilutive securities are excluded from the computation of diluted net loss per share as their inclusion would be anti-dilutive and consist of the following:
| September 30, * | September 30, | |||||||
| 2014 | 2013 | |||||||
| Preferred Stock Series B |
2,700,000 | — | ||||||
| Warrants |
933,617 | 15,596 | ||||||
| Options |
1,225,219 | 163,971 | ||||||
|
|
|
|
|
|||||
| Totals |
4,858,836 | 179,567 | ||||||
|
|
|
|
|
|||||
| * | September 30, 2014 excludes the impact of the 2,700,000 shares underlying the Put Agreements discussed in Note 6 since the Company did not exercise its rights under such agreements until October 17, 2014. |
F-24
COHBAR, INC.
NOTES TO CONDENSED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2014
(unaudited)
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
RECENT ACCOUNTING PRONOUNCEMENTS
In August 2014, the FASB issued a new accounting standard which requires management to evaluate whether there is substantial doubt about an entity’s ability to continue as a going concern for each annual and interim reporting period. If substantial doubt exists, additional disclosure is required. This new standard will be effective for the Company for annual and interim periods beginning after December 15, 2016. Early adoption is permitted. The adoption of this pronouncement is not expected to have a material impact on the Company’s condensed financial statements.
Recent accounting standards that have been issued or proposed by the FASB or other standards-setting bodies that do not require adoption until a future date are not expected to have a material impact on the Company’s condensed financial statements upon adoption.
NOTE 4 – CONVERTIBLE PROMISSORY NOTES PAYABLE
In January 2014, the Company issued Convertible Promissory Notes totaling $210,000 (“January 2014 Notes”). The January 2014 Notes had a maturity date of one year, interest of 0% and included a warrant to purchase an aggregate of 20,946 shares of the Company’s Common Stock at an exercise price of $0.50 per share. The warrants expire the earlier of a liquidation event, upon the effective date of the Company’s initial public offering or in one year. If the January 2014 Notes were not repaid or converted on or prior to the date that is six months after the issuance, the Company was required to issue to the holders of the January 2014 Notes additional warrants equal to the amount of the initial warrants issued. The Company determined the fair value of the warrants issued using the Black-Scholes pricing model, and allocated the proceeds based on the relative fair value of the debt instruments and the related warrants. The aggregate deferred debt discount related to the January 2014 Notes was $137. In April 2014, the January 2014 Notes were converted to shares of the Series B Convertible Preferred Stock (“Series B Preferred Stock”) (see Note 6) and the remaining deferred debt discount was charged to expense.
NOTE 5 – COMMITMENTS AND CONTINGENCIES
LITIGATIONS, CLAIMS AND ASSESSMENTS
In the normal course of business, the Company may be involved in legal proceedings, claims and assessments arising in the ordinary course of business. Such matters are subject to many uncertainties, and outcomes are not predictable with assurance. There are no such matters that are included in the condensed financial statements as of September 30, 2014.
OPERATING LEASE
The Company rents lab space on a month to month basis in Pasadena, California. Rent expense amounted to $5,400 for the three months ended September 30, 2014 and 2013, respectively. Rent expense amounted to $16,200 and $19,800 for the nine months ended September 30, 2014 and 2013, respectively.
F-25
COHBAR, INC.
NOTES TO CONDENSED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2014
(unaudited)
NOTE 6 – STOCKHOLDERS’ DEFICIENCY
AUTHORIZED CAPITAL
In April 2014, the Company amended its Certificate of Incorporation to increase the total number of authorized shares of common stock. The Company, following the amendment, has authorized the issuance and sale of up to 45,000,000 shares of stock, consisting of 37,000,000 shares of common stock having a par value of $0.001 and 8,000,000 shares of Preferred Stock having a par value of $0.001 per share. The holders of Preferred Stock are entitled to receive dividends, when, as and if declared by the Company’s Board of Directors. The dividends on shares of Preferred Stock are cumulative.
As of September 30, 2014, there were no declared but unpaid dividends or undeclared dividend arrearages on any shares of the Company’s capital stock. The holders of Preferred Stock are entitled to be paid out of the assets of the corporation before any payment is made to the holders of common stock in the event of any voluntary or involuntary liquidations, dissolution or winding up of the Company. The Company has reserved 1,025,822 shares of its common stock for the issuance of stock options and restricted stock to its employees, officers, directors and consultants.
CONVERTIBLE PREFERRED STOCK
During the nine months ended September 30, 2014, the Company issued 5,400,000 shares of Series B Preferred Stock in the amount of $2,700,000, net of issuance costs of $86,129, of which $59,920 were incurred during the nine months ended September 30, 2014. 420,000 of these Series B Preferred shares in the amount of $210,000 were issued upon the conversion of the January 2014 Notes (see Note 4). Each share of Series B Preferred Stock is convertible, at the option of the holder, into Common Stock by dividing the Series B original issue price by the Series B conversion price in effect at the time of the conversion. The conversion rate of the Series B Preferred Stock into Common Stock at September 30, 2014, is 1:1. In the event the Company issues additional common stock at any time after the original Series B Preferred Stock issue date, then the Series B conversion price will be adjusted concurrently with such issue. Since the host contract (Series B Preferred Stock) is considered an equity instrument, the embedded conversion option is considered to be closely related to the host and has not been bifurcated from the host contract. The Series B Preferred Stock has a par value of $0.001 and was issued at $0.50 per share. The purchasers of Series B Preferred Stock entered into put agreements requiring the purchasers, at the Company’s option, to purchase from the Company securities of the same type as those sold to investors in any future public offering of the Company’s securities, at the same price as the securities sold in the initial public offering, for an aggregate purchase price of up to $2,700,000. The put agreements expire upon the first occurrence of a change in control or in three years. The Company can exercise its rights under the put agreements beginning on the date the Company first submits an IPO Registration Statement for review by the Securities and Exchange Commission and ending the earlier of the day that is 21 days prior to the effective date of the IPO Registration or the expiration date of the put agreements.
On October 17, 2014, the Company exercised its rights under the aforementioned Put Agreements requiring the purchasers of Series B Preferred Stock to purchase 2,700,000 shares of common stock at the proposed public offering price of $1.00 per share.
F-26
COHBAR, INC.
NOTES TO CONDENSED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2014
(unaudited)
NOTE 6 – STOCKHOLDERS’ DEFICIENCY (CONTINUED)
STOCK OPTIONS
During the nine months ended September 30, 2014, the Company issued an aggregate of 1,061,248 stock options to employees and consultants with an exercise price of $0.26 and a fair value of $0.18 per share. The stock options granted in 2014 are subject to vesting over two to four years and have a term of ten years.
127,532 stock options granted during the nine months ended September 30, 2014, contained performance conditions which included (i) the optionee’s continuous service and (ii) completion of the Company’s initial public offering pursuant to an effective registration statement under the Securities Act of 1933, as amended. Since the stock options contain performance conditions, their fair value has not been recorded since the obligations have not been met as of the date of the financials contained herein.
The following table represents stock option activity for the nine months ended September 30, 2014:
| Weighted Average | ||||||||||||||||||||||||||||
| Stock Options | Exercise Price | Fair Value Vested |
Contractual Life (Years) |
Aggregate Intrinsic Value |
||||||||||||||||||||||||
| Outstanding | Exercisable | Outstanding | Exercisable | |||||||||||||||||||||||||
| Balance – January 1, 2014 |
163,971 | 83,123 | $ | 0.05 | $ | 0.05 | $ | 0.05 | 8.26 | |||||||||||||||||||
| Granted |
1,061,248 | 280,116 | 0.26 | — | — | — | ||||||||||||||||||||||
| Exercised |
— | — | — | — | — | — | ||||||||||||||||||||||
| Cancelled |
— | — | — | — | — | — | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance – September 30, 2014 |
1,225,219 | 393,984 | $ | 0.16 | $ | 0.14 | $ | 0.14 | 9.23 | $ | 34,434 | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
The following table summarizes information on stock options outstanding and exercisable as of September 30, 2014:
| Exercise Price |
Number |
Weighted Average |
Weighted Average |
Number Exercisable |
Weighted Average | |||||
| $0.05 |
163,971 | 7.51 | $0.05 | 113,868 | $0.05 | |||||
| $0.26 |
1,061,248 | 9.23 | $0.26 | 280,116 | $0.26 | |||||
|
|
|
|
|
| ||||||
| Totals |
1,225,219 | 393,984 | ||||||||
|
|
|
WARRANTS
In April 2014, the Company issued 797,075 warrants to its chief executive officer. The warrants have an exercise price of $0.26 and a fair value of $0.21 per warrant. The warrants expire on the earlier of a liquidation event, as defined in the agreement, or in ten years.
In July 2014, the Company issued 100,000 warrants to consultants. The warrants have an exercise price of $0.26 and a fair value of $0.24 per warrant. The warrants expire on the earlier of a liquidation event, as defined, or in five years.
F-27
COHBAR, INC.
NOTES TO CONDENSED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2014
(unaudited)
NOTE 6 – STOCKHOLDERS’ DEFICIENCY (CONTINUED)
WARRANTS (CONTINUED)
The following table represents warrant activity for the nine months ended September 30, 2014:
| Weighted Average | ||||||||||||||||||||||||||||
| Stock Warrants | Exercise Price | Fair Value Vested |
Contractual Life (Years) |
Aggregate Intrinsic Value |
||||||||||||||||||||||||
| Outstanding | Exercisable | Outstanding | Exercisable | |||||||||||||||||||||||||
| Balance – December 31, 2013 |
15,596 | 15,596 | $ | 0.99 | $ | 0.99 | $ | 0.05 | — | |||||||||||||||||||
| Granted |
918,021 | 918,021 | 0.27 | |||||||||||||||||||||||||
| Exercised |
— | — | ||||||||||||||||||||||||||
| Cancelled |
— | — | — | — | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance – September 30, 2014 |
933,617 | 933,617 | $ | 0.28 | $ | 0.28 | $ | 0.21 | 8.89 | $ | — | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
The Company recorded $17,262 and $5,399 of stock based compensation in the three months ended September 30, 2014 and 2013, respectively. The Company recorded $239,760 and $15,015 of stock based compensation in the nine months ended September 30, 2014 and 2013, respectively. The compensation expense associated with stock-based awards granted to individuals is recorded by the Company in the same expense classifications as the cash compensation paid to those same individuals.
NOTE 7 – ACCRUED EXPENSES
Accrued expenses at September 30, 2014 and December 31, 2013 consist of:
| 2014 | 2013 | |||||||
| Lab services |
$ | 45,049 | $ | — | ||||
| Professional fees |
152,525 | 32,284 | ||||||
| Consultant fees |
47,500 | 33,250 | ||||||
| Interest |
9,103 | 4,003 | ||||||
| Expense reimbursement |
5,000 | 98 | ||||||
|
|
|
|
|
|||||
| $ | 259,177 | $ | 69,635 | |||||
|
|
|
|
|
|||||
NOTE 8 – RELATED PARTY
The Company is a party to consulting agreements with two of its Board members. During the nine months ended September 30, 2014 and 2013, the Company recorded $21,708 and $9,000, respectively, for services performed by each Board member. As of September 30, 2014, no consulting fees were due and payable to these Board members.
NOTE 9 – SUBSEQUENT EVENTS
On November 20, 2014, the Company increased the aggregate number of shares of its common stock that may be issued pursuant to stock awards. The maximum number of shares of common stock that may be issued pursuant to stock awards is 2,616,041, an increase of 365,000 shares.
F-28
COHBAR, INC.
NOTES TO CONDENSED FINANCIAL STATEMENTS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2014
(unaudited)
NOTE 9 – SUBSEQUENT EVENTS (CONTINUED)
On November 20, 2014, the Company granted 1,475,687 stock options to directors, employees and consultants with an exercise price of $0.73. The stock options contained four year vesting schedules per option grant and have a term of ten years.
The Company evaluates events that have occurred after the balance sheet date but before the financial statements are issued. Based upon the evaluation, the Company did not identify any recognized or non-recognized subsequent events that would have required adjustment or disclosure in the condensed financial statements, except as disclosed.
F-29
 Mitochondrial-Derived Peptides (MDPs) -
A New Source of Therapies for Diseases of Aging
Initial Public Offering | •, 2014
An amended and restated preliminary prospectus containing important information relating to
the securities described in this document has been filed with the securities regulatory
authorities in each of the provinces of Canada, other than Quebec. A copy of the amended and restated preliminary prospectus, and
any amendment, is required to be delivered with this document. The amended and restated
preliminary prospectus is still subject to completion. There will not be any sale or
any acceptance of an offer to buy the securities until a receipt for the final prospectus has been issued. This document does not provide full
disclosure of all material facts relating to the securities offered. Investors should read the
amended and restated preliminary prospectus, the final prospectus and
any
amendment
for
disclosure
of
those
facts,
especially
risk
factors
relating
to
the
securities
offered,
before
making
an
investment
decision.
Annex A
Roadshow Marketing Materials |
 Legal
Disclaimer A-2
An investment in the securities described in this presentation is subject to a number of risks that
should be considered by a prospective purchaser. Prospective purchasers should carefully
consider the risk factors described under “Risk Factors” and “Forward-Looking Statements” included in the amended and restated preliminary prospectus of CohBar, Inc. (the
“Company”), dated November 28, 2014, including the U.S. prospectus forming a part thereof
(the “preliminary prospectus”) before purchasing securities described hereunder.
Prospective purchasers should rely only on the information contained in the preliminary prospectus.
This presentation is qualified in its entirety by reference to, and must be read in conjunction
with, the detailed information appearing in the preliminary prospectus. Neither the Company nor any of the Agents have authorized anyone to provide prospective
purchasers with different or additional information. The Company is not offering, or soliciting offers
to acquire, the Offered Units described in this presentation in any jurisdiction in which the
offer is not permitted. Prospective purchasers should not assume that the information contained in this presentation is accurate as of any date other than the date of the
preliminary prospectus, or where information is stated to be as of a date other than the date of the
preliminary prospectus, such other applicable date. For prospective purchasers outside Canada,
neither the Company nor the Agents have done anything that would permit the offering or distribution of this document together with the preliminary prospectus in any
jurisdiction where action or that purpose is required, other than in Canada. No offer or sale of Units
will be made in the United States or any state, district, commonwealth or territory thereof.
Although this preliminary prospectus contains a registration statement on Form S-1 filed with the United States Securities and Exchange Commission (the “SEC”) such
registration statement has not yet been declared effective and the Units will not be offered or sold
in the United States or any state, district, commonwealth or territory thereof, nor will offers
or sales of the Units be made to any person who is a “U.S. person” as defined in Rule 902(k) of Regulation S promulgated under the United States Securities Act of 1933, as
amended (the “Securities Act”). Prospective purchasers are required to inform themselves
about and to observe any restrictions relating to the Offering and the distribution of this
presentation and of the preliminary prospectus. In this presentation,
all amounts are in United States dollars, unless otherwise indicated. Terms undefined herein have the meanings ascribed to them in the preliminary prospectus.
Some of the statements contained in this presentation including, without limitation,
financial and business prospects and financial outlooks, may be forward-looking statements which
reflect management’s expectations regarding future plans and intentions, growth, results of
operations, performance and business prospects and opportunities. Words such as “may”,
“will” “should”, “could”, “anticipate”, “believe”,
“expect”, “intend”, “plan”, “potential”, “continue” and similar expressions have been used to identify these forward looking statements.
Examples of such forward-looking statements within the preliminary prospectus include: statements
regarding anticipated outcomes of research, pre-clinical and clinical trials for the
Company’s lead peptides and other MDPs; expectations regarding the future market for any drug the
Company may develop; expectations regarding the growth of MDPs as a significant future class of
drug products; statements regarding the anticipated therapeutic properties of drug development candidates derived from MDPs; expectations regarding the
Company’s ability to effectively protect its intellectual property; statements concerning
perceived competitive advantages and the Company’s ability to defend competitive advantages;
and expectations regarding the Company’s ability to attract and retain qualified employees and
key personnel. These statements reflect management’s current beliefs and are based on information currently
available to management. Forward-looking statements involve significant risks and
uncertainties, including without limitation, those listed in the “Risk Factors” section of
the preliminary prospectus. A number of factors could cause actual results to differ materially from
the results discussed in the forward-looking statements including, but not limited to, changes in
general economic and market conditions and the risk factors disclosed under “Risk
Factors” in the preliminary prospectus. Although the forward-looking statements contained in
this presentation are based upon what management believes to be reasonable assumptions,
management cannot assure that actual results will be consistent with these forward-looking statements. Investors should not place undue reliance on forward-looking
statements. These forward-looking statements are made as of the date hereof and we assume no
obligation to update or revise them to reflect new events or circumstances, except as required
by applicable laws. The forward-looking statements contained in this presentation are expressly qualified by the
foregoing cautionary statements. An investment in Offered Units is suitable for only those
purchasers who are willing to risk a loss of their entire investment and who can afford to lose their
entire investment. Purchasers should read this entire preliminary prospectus and consult their
own professional advisors to assess the income tax, legal and other aspects of an investment in Offered Units.
The information contained on the Company’s corporate website is not intended to be included in or
incorporated by reference into this presentation or the preliminary prospectus and purchasers
should not rely on such information when deciding whether or not to purchase Offered Units.
CohBar, Inc. |
 CohBar, Inc.
The Company
CohBar,
Inc.
(“CohBar”
or
the
“Company”)
is
a
biotechnology
company
whose
mission
is
to treat age-related diseases and extend healthy life span through the discovery of novel
mitochondrial-derived peptides ("MDPs") and the advancement of their
development into clinically relevant and commercially successful therapeutics.
CohBar is a first mover in exploring the mitochondrial genome to
identify MDPs with
potential to be developed into transformative medicines.
A-3
Presentation Overview
•
The Team: Founders, Management, Directors
•
Centenarian Research and Discoveries
•
Mitochondrial Biology and Genomics
•
MDP Research and Discoveries
•
Diseases and Targets
•
CohBar Objectives and Strategy
•
Public Company Benchmarks, Offering/Use of Proceeds and Investment Highlights
|
 CohBar, Inc.
The Founders
Pinchas Cohen, MD
•
Dean of the Davis School of Gerontology at the University of Southern California
•
Executive Director of the Ethel Percy Andrus Gerontology Center
•
William and Sylvia Kugel Dean’s Chair in Gerontology
•
Recipient of numerous awards for research, including the National Institute of Aging
“EUREKA”-Award,
the
NIH-Director-Transformative
RO1-Grant
and
the
Glenn
Award
for
Research in Biological Mechanisms of Aging
•
Dr.
Cohen
holds
an
MD
degree
from
the
Technion
Israel
Institute
of
Technology
•
Postdoctoral training at Stanford University
Nir Barzilai, MD
•
Dr. Barzilai is the Director of: the Institute for Aging Research, the Paul F. Glenn Center
for Biology of Human Aging Research and the NIH Nathan Shock Center of Excellence in
the Basic Biology of Aging
•
Recipient of the Beeson Fellowship for Aging Research, the Ellison Medical
Foundation Senior Scholar
in
Aging
Reward,
the
Paul
F.
Glenn
Foundation
Award,
the
NIA
Nathan
Shock
Award
and the 2010 Irving S. Wright Award of Distinction in Aging Research
•
Dr. Barzilai holds an MD degree from the Technion Israel Institute of Technology
John Amatruda, MD
•
Former SVP and Franchise Head for Diabetes and Obesity at Merck Research Laboratories where
he lead the development and regulator approvals of Januvia and Janumet for Type 2
Diabetes •
Previously Dr. Amatruda founded and managed a drug discovery group at Bayer Corporation,
where he was VP and Therapeutic Area Research Head for Metabolic Disorders
research David Sinclair, PhD
•
Professor in the Genetics Department at Harvard Medical School, Co-Director of the Paul
F. Glenn Laboratories for Biological Mechanisms of Aging and a Professor in the
Physiology and Pharmacology Department at the University of New South Wales
•
Co-founder of Sirtris Pharmaceuticals (NASDAQ:SIRT) and Genocea Biosciences
(NASDAQ:GNCA) A-4 |
 CohBar, Inc.
Management Team
Jon Stern, MBA
Chief Executive Officer
•
Senior business executive with over 30 years of diversified management experience
•
COO of The Key Worldwide, EVP of Integrated China Media and VP of IMC, a
division of Kaufman and
Broad, CEO and Founder of Cine Coasters, Inc., acquired by division of
Liberty Media
•
B.S. in Business Administration from The University of California, Berkeley
•
MBA from Marshall School of Business at the University of Southern California
Kenneth Cundy, PhD
Chief Scientific Officer
•
Joined CohBar in November 2014 as Chief Scientific Officer (CSO)
•
CSO and SVP for Xenoport, Inc. (NASDAQ:XNPT)
•
Senior director of biopharmaceutics at Gilead Sciences
•
Principal research investigator at Sterling Drug, a division of Eastman Kodak
•
B.S. in pharmacy from the University of Manchester
•
Registered as a pharmacist in the UK
•
Ph.D. in pharmaceutical sciences from the University of Kentucky
•
Postdoctoral training in biochemistry at the University of California, Berkeley
Jeffrey Biunno, CPA
Chief Financial Officer
•
25 years of experience with small, medium and large capitalization companies
•
CFO of Manage IQ (acquired by Red Hat)
•
VP & Controller of Dialogic and VP & Controller of Novadigm, Inc. (NASDAQ:NVDM)
•
MBA from Montclair State University
•
Certified Public Accountant, licensed in the State of New Jersey
A-5 |
 CohBar, Inc.
Board of Directors
Albion Fitzgerald
Chairman
•
Over 45 years of experience in the technology sector
•
Member of the board of directors since May 2014 and appointed chairman in July 2014
•
Previously CEO and Chairman of ManageIQ, Inc., Co-Founder, CEO and Chairman of
Novadigm, Inc. ( NASDAQ: NVDM) and Founder and CEO of Telemetrix, Inc.
Marc Goldberg
Director
•
Joined the board of directors in November 2014 and Managing Director at BioVentures
Investors (life science focused venture and private equity investment firm)
•
Previously on the board of directors of Enanta Pharmaceuticals (NASDAQ: ENTA), President
& CEO of the Massachusetts Biotechnology Research Institute, founding President of
the Massachusetts Biotechnology Council and VP, Finance & Corporate Development,
CFO and Treasurer of Safer, Inc.
•
AB (Harvard), JD (Harvard Law School) and MBA (Harvard Business School)
Jon Stern
Director
•
Joined the board of directors in May 2014
Nir Barzilai, MD
Director
•
Co-founder and has served on the board of directors since 2009
Pinchas Cohen, MD
Director
•
Co-founder and has served on the board of directors since 2009
A-6 |
 CohBar, Inc.
Centenarians and Their Offspring
A-7
The potential relevance of humanin in longevity and the prevalence of aging related
diseases has been observed in studies of centenarians (people living to 100 years old)
and their offspring.
•
Centenarians have less or similar
prevalence of diseases compared
to people who are 20 to 30 years
younger.
•
Their offspring have less prevalence of
diseases compared to their age group.
Disease Prevalence within Population
0
5
10
15
20
25
30
35
40
Diabetes
Mellitus
(%)
Myocardial
Infarction
(%)
Stroke
(%)
Hypertension (%)
P
O
C
**
**
**
**
Centenarians
Offspring
Control
** p<0.01 |
 Exceptional
Health and Humanin
A-8
The development of assays to measure humanin levels in plasma enabled the
observation of humanin levels in aged patients and patients with
poor endothelial
function associated with cardiovascular diseases.
•
Humanin declines with age but is higher in offspring of centenarians when
compared to an age and gender matched control group.
•
Humanin is lower in humans with poor endothelial function, a major risk factor for
cardiovascular diseases.
Offspring with Familial Exceptional Longevity
Humans with Normal Endothelial Function
Control
0
100
200
300
400
500
600
700
Humanin (pg/ml)
P<0.03
Offspring
0
500
1000
1500
2000
Poor
Humanin (pg/ml)
Normal
P<0.03
Endothelial Function
CohBar, Inc. |
 CohBar, Inc.
Mitochondrial Biology and Genomics
Mitochondria are components within the cell that produce energy and regulate cell
death in response to signals received from the cell.
A-9
Mitochondria are the only cell components besides the
nucleus that have their own DNA.
Until recently, scientists believed the mitochondrial
genome contained only 37 genes.
Research by our founders has revealed that the
mitochondrial genome has as many as 80 distinct new
genes that encode peptides (small amino acid chains),
which we refer to as Mitochondrial-Derived Peptides,
or “MDPs.”
Mitochondrial Genomics: |
 CohBar,
Inc. MDPs influence cellular activities by acting as messengers
between cells, triggering intra-cellular changes
MDPs have metabolic effects, neuro-protective effects, cyto-protective
effects and anti-inflammatory effects
Humanin was the first MDP discovered in 2001 by Dr. Cohen (CohBar co-founder)
and others
Humanin
has
protective
effects
in
various
animal
disease
models,
including
Alzheimer’s disease, atherosclerosis, myocardial and cerebral ischemia, and
Type 2 Diabetes
A-10
MDP’s are a diverse and largely unexplored collection of peptides which has the
potential to lead to novel therapeutics for a number of diseases
with significant
unmet medical needs.
MDP Biological Effects:
MDPs –
A New Untapped Field in Biology |
 CohBar,
Inc. CohBar Founders’
CohBar Founders’
MDP Discoveries
MDP Discoveries
The Company’s research to date is focused on discovering and evaluating our MDPs for
potential development as drug candidates. CohBar’s current focus is on the
following MDPs:
1.
MOTS-c
–
CohBar’s
studies
indicate
that
MOTS-c
plays
a
significant
role
in
regulation
of metabolism and the Company believes a MOTS-c analog has therapeutic
potential for Type 2 Diabetes and as well as other diseases such as obesity, fatty
liver and certain cancers.
2.
SHLP-6
–
CohBar’s investigational research of SHLP-6 and its potential to treat
cancer has advanced. SHLP-6 cancer treatment models have demonstrated
suppression of cancer progression in mice.
3.
SHLP-2
–
In vitro experiments have shown SHLP-2 to have protective effects against
neuronal toxicity and the Company believes a SHLP-2 analog may be useful in the
treatment of Alzheimer's disease.
4.
Humanin
–
The first MDP to be discovered has demonstrated benefits in a rat
model of Type 2 Diabetes.
A-11 |
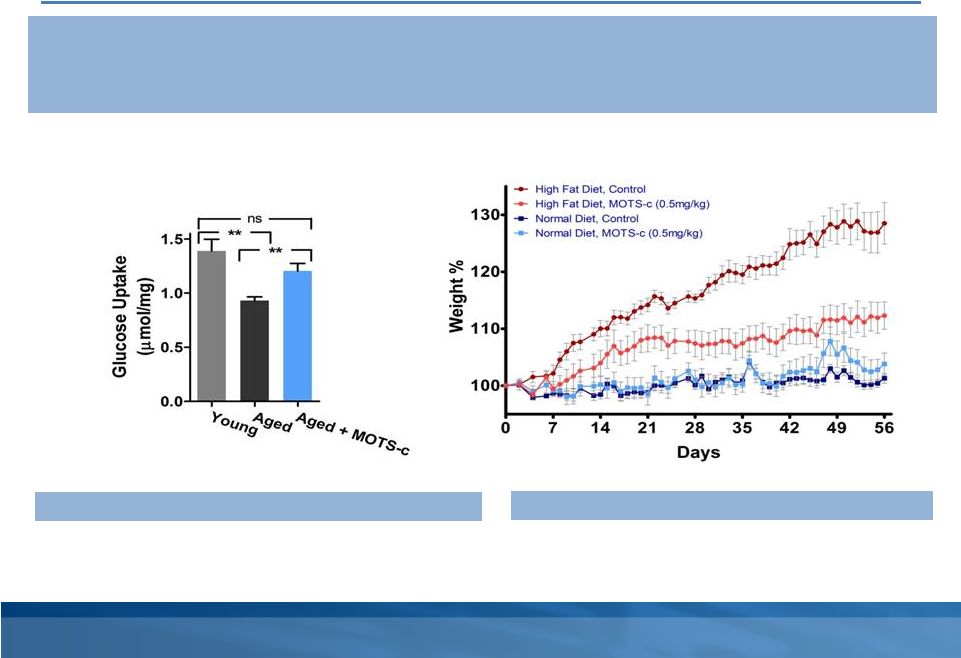 CohBar,
Inc. CohBar MDP –
MOTS-c
A-12
MOTS-c reverses age-dependent insulin resistance
MOTS-c prevents weight gain in mice
The Company plans to advance research on MOTS-c and its analogs as our lead MDP
program
which
we
believe
has
the
greatest
potential
for
development
as
a
commercially
successful drug.
Soleus Muscle 2-DG Uptake
(3 mo. Vs 12 mo. Mice) |
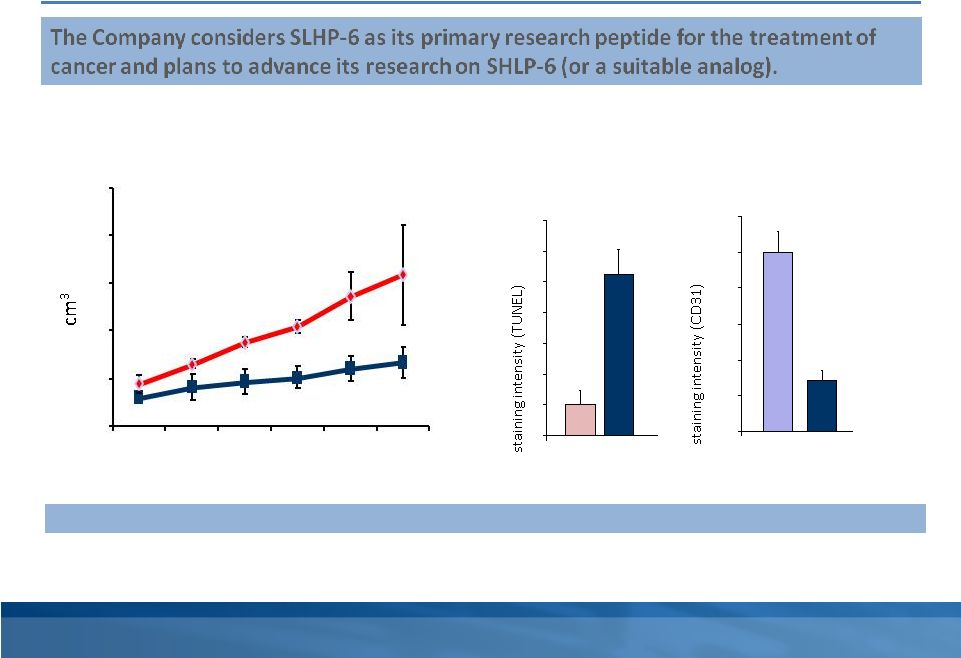 CohBar,
Inc. CohBar MDP –
SLHP-6
A-13
0
0.2
0.4
0.6
0.8
1.0
1.2
Control
P
= 0.004
0
1
2
3
4
5
6
7
Control
SHLP-6
P
= 0.03
Increased
Cancer Cell
Death
Reduced Tumor
Blood Vessel
Development
SHLP-6
* P< 0.01
Injection day
*
*
*
*
*
SHLP-6
Control
Scrambled
Peptide
0
1
2
3
4
5
1
2
3
4
5
6
Reduced Tumor Growth
SHLP-6 |
 CohBar,
Inc. CohBar MDPs –
Diseases and Patents
A-14
Granted /
Filed
Composition
Claims
Type 1
Diabetes
Type 2
Diabetes
Obesity
Fatty Liver
Cancer
Alzheimer’s
Atherosclerosis
MOTS-c
Filed
SHLP-6
Filed
SHLP-2
Granted
Humanin
Analogs
Granted
Humanin
Analogs
Two
Granted
Humanin &
Humanin
Analogs
Filed
Therapeutic Activities / Method of Use Claims
CohBar
is
the
exclusive
licensee
from
the
Regents
of
the
University
of
California
and
the
Albert
Einstein College of Medicine to four issued US patents and four US and
international patent applications directed to compositions comprising MDPs
and MDP analogs and methods of their use in the treatment of indicated
diseases. The Company has developed an IP position and operational plan to allow for
proprietary exploitation and protection of its drug candidates: |
 CohBar,
Inc. CohBar’s Targets -
Large medical needs
The Company’s drug discovery efforts are centered on the identification of MDPs that
have therapeutic potential to be advanced as drug candidates for these major
diseases: Type
2
Diabetes
–
The
World
Health
Organization
(“WHO”)
reports
that
over
346
million
people worldwide suffer from diabetes, of which 90% is Type 2 Diabetes.
Cancer
–
WHO
estimates
that
in
2012,
there
were
14.1
million
new
cancer
cases
diagnosed, 8.2 million cancer deaths and 32.6 million people living with cancer
worldwide.
Alzheimer's Disease
–
The Alzheimer’s Association®
reports that an estimated 5.2 million
Americans suffered from Alzheimer’s disease in 2013, and that by 2025 an estimated 7.1
million Americans will be afflicted by the disease, a 40 percent
increase from currently
affected patients.
Atherosclerosis
–
Atherosclerosis is commonly referred to as a “hardening”
or furring of
the arteries. This process is the major underlying risk for developing heart attacks.
A-15 |
 CohBar,
Inc. CohBar Objectives
A-16
Selection of an MDP drug candidate:
•
Existing MDPs and any newly discovered MDPs
•
Initial evaluation in efficacy models
•
Prioritization of potential lead molecules
•
Synthesis of new analogs
•
Iterative evaluation of stability, pharmacokinetics, and efficacy
•
Selection of a candidate for IND-enabling activities
Completion of IND-enabling activities:
•
Preclinical testing (toxicology, safety pharmacology, genetic toxicity,
pharmacokinetics)
•
GMP manufacturing of drug substance and formulation
•
Filing and clearance of an Investigational New Drug (IND) application
with the FDA to allow subsequent clinical trials |
 CohBar,
Inc. CohBar’s Strategy
To build a multi-product company based on our expertise in MDP biology that,
independently or together with strategic partners, discovers, develops and
commercializes first-
and best-in-class medicines to treat a wide variety of diseases with
large unmet medical need.
A-17
•
Maintaining our first mover advantage in MDP therapeutics
•
Exploiting our MDP discoveries to date by advancing research and
development and
expanding our pipeline of research peptides
•
Expanding our intellectual property portfolio of patents and licenses
•
Leveraging relationships with academic partners and contract research
organizations (CROs)
•
Effectively utilizing research loans and government grants (for example, the award
of a research loan from the Alzheimer’s Drug Discovery Foundation)
•
Developing strategic partnerships with larger pharmaceutical companies to support
our research programs, future development and commercialization efforts
|
 CohBar,
Inc. Therapeutic Drug Development
Public Company Market Cap Benchmarks
A-18
In
accordance
with
Section
13.7(4)
of
National
Instrument
41-101
–
General
Prospectus
Requirements,
all
the
information relating to the Company’s comparables and any disclosure relating to the
comparables, which is contained in the live presentation to be provided to potential
investors, has been removed from this template version for purposes of its filing on
the System for Electronic Document Analysis and Retrieval (SEDAR).
|
 CohBar,
Inc. Summary of Offering and Use of Proceeds
Summary of Offering
Issuer:
CohBar, Inc.
Offering Price:
$1.00 per unit.
Gross Proceeds:
$11,250,000, minimum.
Units Offered:
11,250,000 units, each consisting of one common share and one half of one common
share purchase warrant. Each whole warrant will entitle its holder to
purchase one common share at an exercise price of $2.00 per share at any
time for 24 months after the closing of this offering, provided, however, that if the volume weighted average
trading price of the common shares equals or exceeds $3.00 per share for 20
consecutive trading days after the date on which the common shares are
first traded on the TSX-V, the Company shall have the right and option,
exercisable
at
its
sole
discretion,
to
accelerate
the
expiration
time
of
the
Warrants
on
30
days
prior
notice.
Principal Shareholders:
Upon
closing
of
the
offering,
Dr.
Pinchas
Cohen
will
hold
16.87%
of
the
outstanding
shares
and
Dr.
Nir
Barzilai
will
hold 15.62% of the outstanding shares.
Lock-Up Agreements:
180 days, subject to certain limited exceptions.
Listing:
An application has been made to the TSX-V
Closing:
On or about •
Funds Available:
Estimated working capital as of December 31, 2014
Proceeds from concurrent issuance of units pursuant to the exercise of our Put
Rights Estimated Net proceeds from the Offering
$440,000
$2,700,000
$10,212,500
Total
$13,352,500
Use of Proceeds:
The Company intends to use the proceeds described above as follows:
A-19
~$10.25 million to fund research, development and pre-clinical testing
activities, including costs associated with expansion of the Company’s
internal scientific leadership and staff, lab facilities, equipment and supplies
and external contract research services;
~$3.0 million to fund general and administrative expenses, including increased
legal, accounting, insurance and other administrative expenses associated
with being a publicly traded company; and ~$100 thousand for general working
capital. |
 CohBar,
Inc. Investment Highlights
A-20
Our founders are scientific experts and thought leaders at the intersection of
cellular and mitochondrial genetics and biology, the biology of aging, metabolism,
and drug discovery, development and commercialization.
Scientific research underlying our founder’s discoveries and CohBar’s IP portfolio
was conducted by Dr. Cohen, Dr. Barzilai and their academic collaborators with
the support of research grants aggregating over $30 million.
CohBar is a first mover in exploring the mitochondrial genome to
identify MDPs
with potential to be developed into transformative medicines that could address
significant unmet medical needs.
Given the age-related risk factors associated with these disease indications, an
effective therapeutic drug could offer substantial improvements in the quality of
life, longevity, and medical and care cost burden of our aging population.
|
CERTIFICATE OF COHBAR, INC.
Dated December 18, 2014
This prospectus constitutes full, true and plain disclosure of all material facts relating to the securities offered by this prospectus as required by the securities legislation of each of the Provinces of Canada other than Quebec.
| By: (Signed) “Jon Stern” Chief Executive Officer |
By: (Signed) “Jeffrey F. Biunno” Chief Financial Officer |
On behalf of the Board of Directors of Cohbar, Inc.
| By: (Signed) “Albion J. Fitzgerald” Chairman |
By: (Signed) “Pinchas Cohen” Director |
CDN-C-1
CERTIFICATE OF THE AGENT
Dated December 18, 2014
To the best of our knowledge, information and belief, this prospectus constitutes full, true and plain disclosure of all material facts relating to the securities offered by this prospectus as required by the securities legislation of each of the Provinces of Canada other than Quebec.
Haywood Securities Inc.
By: (Signed) “Lawrence Rhee”
CDN-C-2